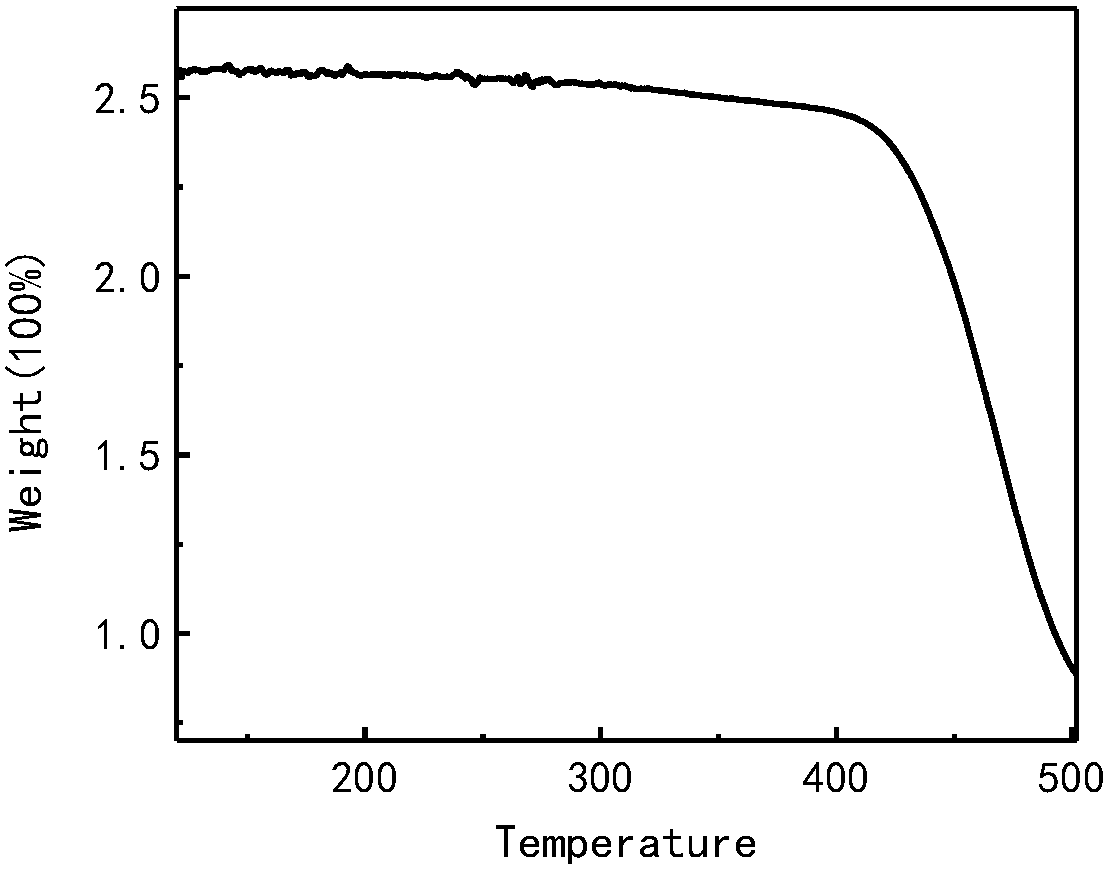
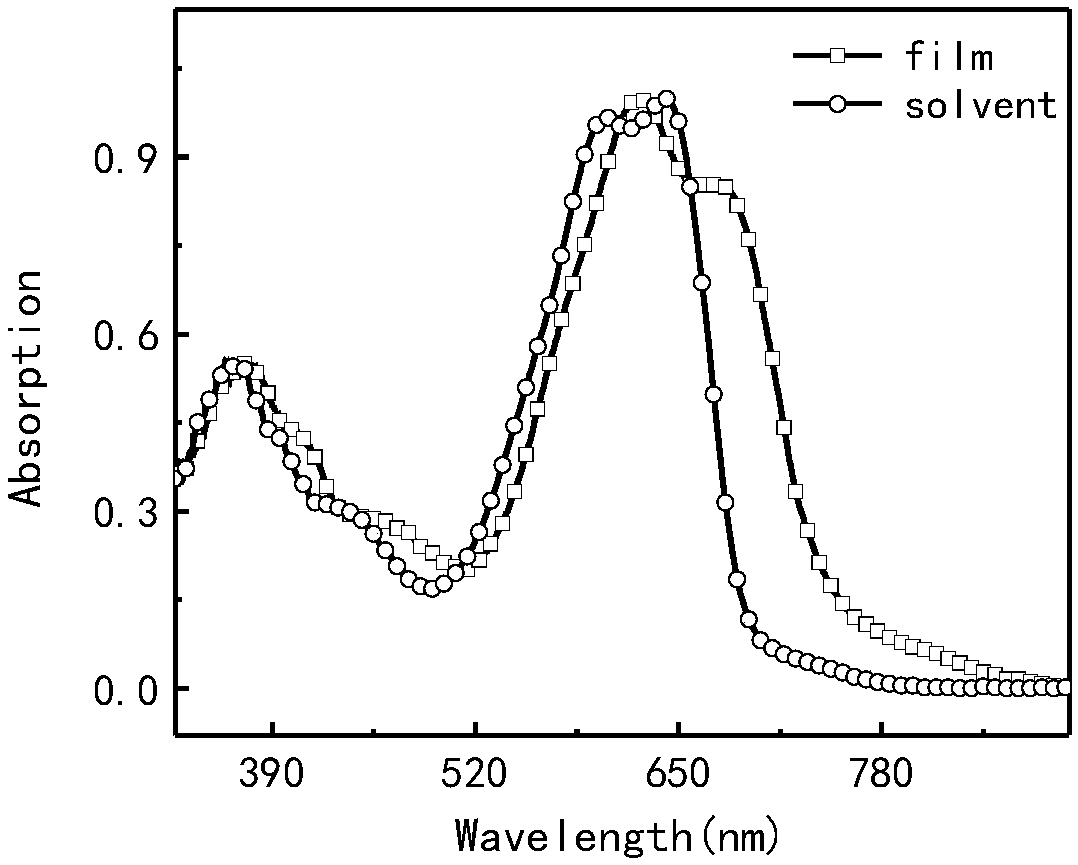
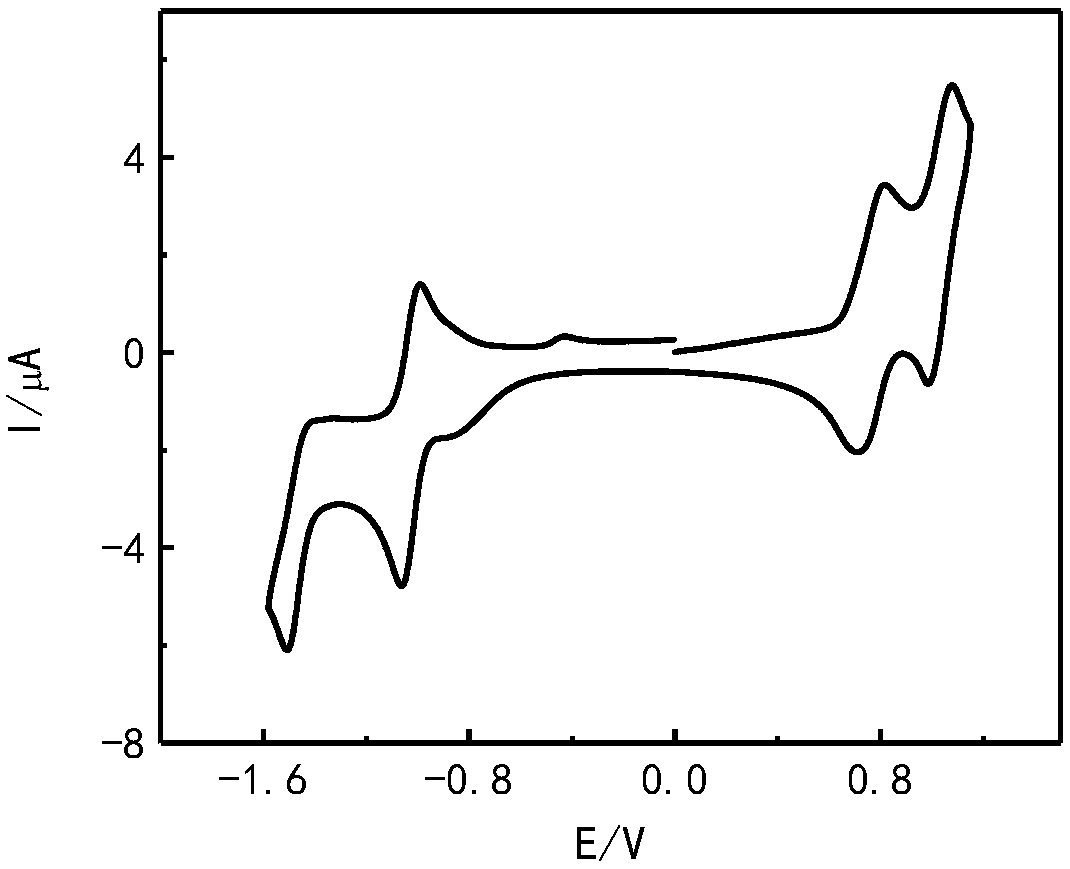
Preparation method and application of a class of organic semiconductor materials based on polycyclic aromatic hydrocarbon k-position and imidazolyl groups
An organic semiconductor and polycyclic aromatic hydrocarbon technology, which is applied in the field of preparation of polycyclic aromatic hydrocarbon K-position imidazole-based organic semiconductor materials, can solve the problems that polycyclic aromatic hydrocarbon materials are rarely used in organic solar cells and the like, and is conducive to molecular stacking, The effect of short synthesis route and huge application prospect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1. In a single-necked round-bottom flask (250 mL), phenanthrenequinone (3 g, 14 mmol), ammonium acetate (13.98 g, 181 mmol) and ethanol (100 mL) were added, and stirred at 78°C under reflux. Then, 2-thiophenecarbaldehyde (1.6 g, 14 mmol) was added to the reaction system. After the addition is complete, continue stirring at 78 degrees overnight. After the reaction, the ethanol was spin-dried, the solid was washed with water, filtered with suction, dried, and separated and purified on a silica gel column to obtain 1.48g of 2-thienyl-1H-phenanthroline [9,10-d]-imidazole (hereinafter referred to as TPh), yield 34.3%. 1H NMR (400MHz, DMSO) δ (ppm): 8.84 (d, J = 14.1, 8.3 Hz, 2H), 8.48 (d, J = 21.5, 7.7 Hz, 2H), 7.92 (d, J = 3.6 Hz, 1H ), 7.79-7.67 (m, 3H), 7.62 (d, J=6.7 Hz, 2H), 7.29-7.24 (m, 1H), 5.74 (s, 1H).
[0034] Add TPh (1.6g, 5.3mmol), potassium carbonate (3.68g, 26.7mmol), potassium iodide (0.044g, 0.3mmol) and N,N-dimethylformamide (hereinafter referred t...
Embodiment 2
[0038] Example 2. In a single-neck round bottom flask (250 mL), pyrene dione (4.5 g, 19 mmol), ammonium acetate (18.8 g, 244 mmol) and ethanol (100 mL) were added, and the mixture was refluxed and stirred at 78 degrees. Then, 2-thiophenecarbaldehyde (2.18 g, 19 mmol) was added to the reaction system. After the addition is complete, continue stirring at 78 degrees overnight. After the reaction, the ethanol was spin-dried, the solid was washed with water, filtered with suction, dried, and separated and purified on a silica gel column to obtain 2.15g of 10-(2-thienyl)-9H-pyrene[4,5-d]imidazole (hereinafter referred to as TPy). The yield was 34.2%. 1H NMR(400MHz,DMSO)δ(ppm): 8.76(d,J=7.5Hz,1H), 8.70(d,J=7.6Hz,1H), 8.26-8.08(m,7H),7.98(d,J = 3.6 Hz, 1H), 7.74 (d, J = 5.0 Hz, 1H), 7.32-7.28 (m, 1H).
[0039] Add TPy (2.0g, 6.2mmol), potassium carbonate (4.3g, 30.9mmol), potassium iodide (0.05g, 0.3mmol) and N,N-dimethylformamide (hereinafter referred to as DMF) (10 mL), stirred at ...
Embodiment 3
[0043] Example 3. TPh (1.0g, 3.3mmol), potassium carbonate (2.3g, 16.7mmol), potassium iodide (0.028g, 0.17mmol) and N,N-dimethylform were added to a two-necked round bottom flask (100mL) Amide (hereinafter referred to as DMF) (10 mL), stirred at room temperature. Then 1-bromooctane (0.83 g, 4.3 mmol) was added dropwise. After the addition is complete, react at room temperature overnight. After the reaction, it was extracted with water and dichloromethane, concentrated, and separated and purified by silica gel column to obtain 1.1g 1.1g of 1-octyl-2-thienyl-1H-phenanthrene[9,10-d]imidazole (hereinafter referred to as TPh-C8) ). The yield is 80%. 1H NMR(400MHz,CDCl3)δ(ppm)8.86-8.82(m,1H), 8.80(d,J=7.8Hz,1H), 8.69(d,J=8.3Hz,1H), 8.29-8.23(m, 1H), 7.74–7.60 (m, 4H), 7.58–7.51 (m, 2H), 7.23 (dd, J = 5.1, 3.6 Hz, 1H), 4.76–4.69 (m, 2H), 2.08 (d, J = 15.6, 7.8 Hz, 2H), 1.63 (s, 1H), 1.50-1.39 (m, 2H), 1.39-1.21 (m, 9H), 0.88 (d, J = 8.8, 5.0 Hz, 3H).
[0044] In a reaction flask ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com