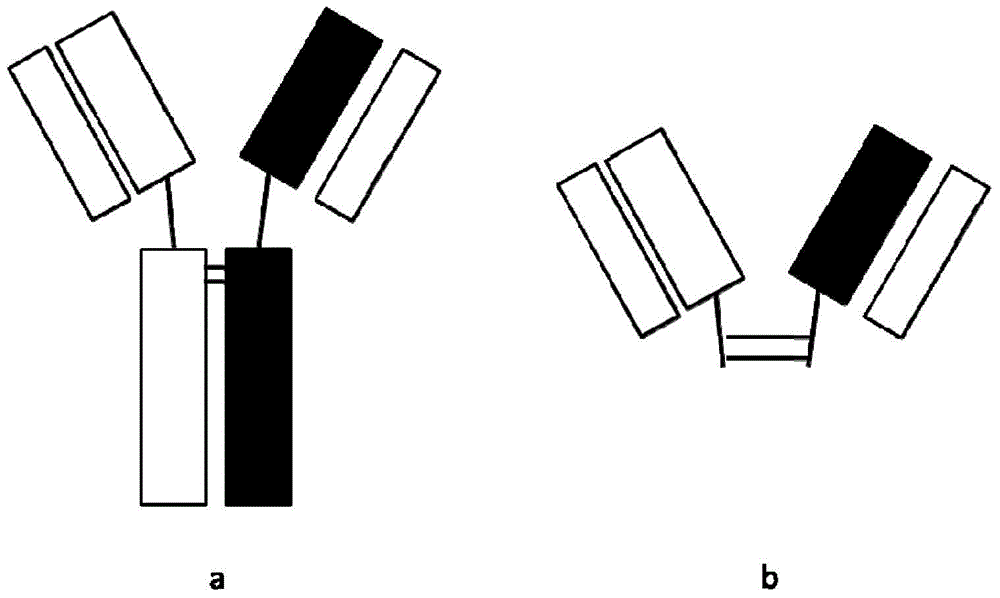
Bispecific antibody or antibody mixture having common light chain
A bispecific antibody and common light chain technology, applied in the direction of antibodies, translation products of oncogenes, anti-receptors/cell surface antigens/cell surface determinant immunoglobulins, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0157] Embodiment 1 Acquisition of common light chain
[0158] 1. Sequence and structure acquisition
[0159] Obtain the complex crystal structure of Trastuzumab and Pertuzumab from the Protein Data Bank (PDB, www.pdb.org), the PDB number of Trastuzumab is 1N8Z, and the PDB number of Pertuzumab is 1S78 . Two screening strategies can be used to identify amino acid contacts between CH3-CH3: (i) distance of amino acid interactions (ii) solvent accessible region analysis. Here, screening is performed based on the interaction distance of amino acids.
[0160] 2. Monoclonal antibody light chain and antigen HER2 interface amino acid acquisition
[0161] According to the amino acid contact rule, interfacial amino acids refer to those amino acids whose distance between the heavy atoms of the side chain and the heavy atoms of any amino acid of another chain is less than a threshold value. Here the threshold is chosen as In some literature it is also possible to choose (Bahar and...
Embodiment 2
[0190] Example 2 Preparation and functional verification of antigenic protein HER2 variant protein
[0191] 1. Design of HER2 variant protein that only binds Pertuzumab
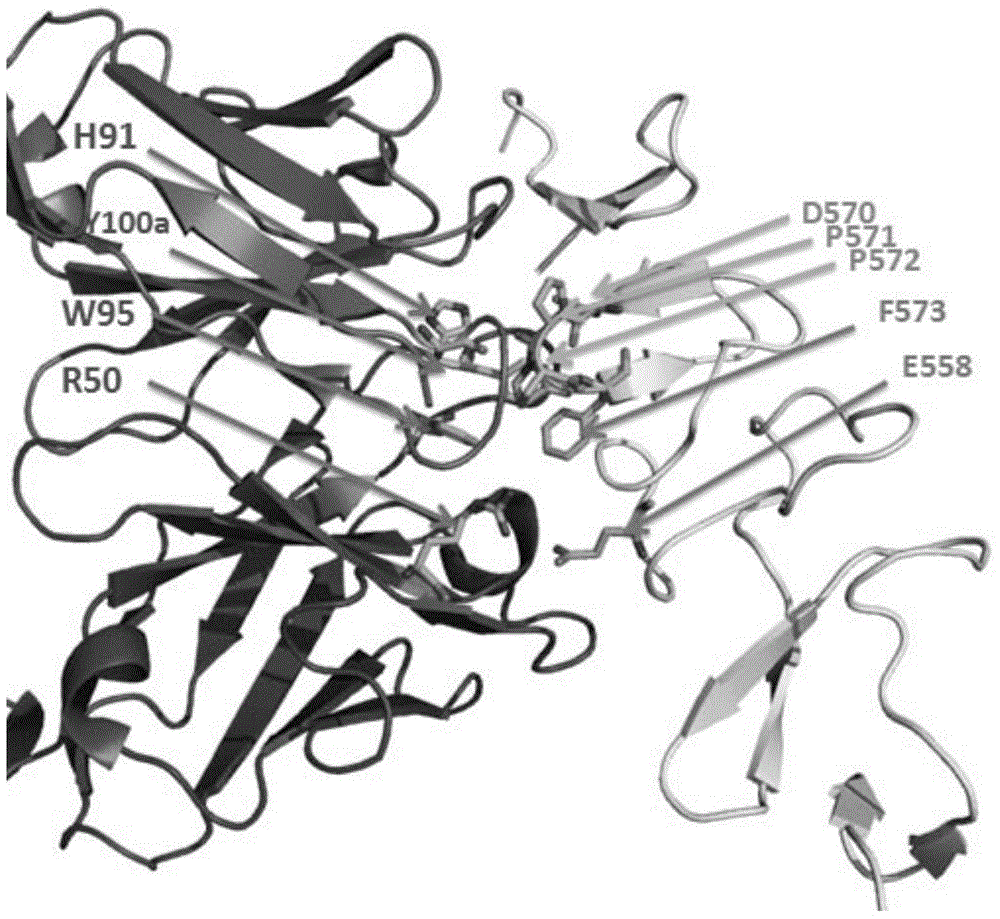
[0192] RobertF.Kelley'andMarkP.O'Connell announced in 1993 the kinetic parameters of the binding of trastuzumab and corresponding mutations to the extracellular domain (ECD) of HER2. Among them, H91A, R50A, W95A, and Y100aA have a significant impact on the binding of trastuzumab to the extracellular domain of HER2. The Hyun-SooCho team crystallized the complex of trastuzumab Fab fragment and HER2 extracellular domain (ECD) in 2003 (PDB number: 1N8Z), and the results were published in Nature. By analyzing the structure of the complex of trastuzumab Fab fragment and HER2 extracellular domain (ECD) (its sequence is shown in SEQ ID NO: 18), we obtained the trastuzumab Fab fragment and HER2 extracellular domain (ECD ) interface with amino acids, as shown in Table 5.
[0193] Focus on the analysis of the amino a...
Embodiment 4
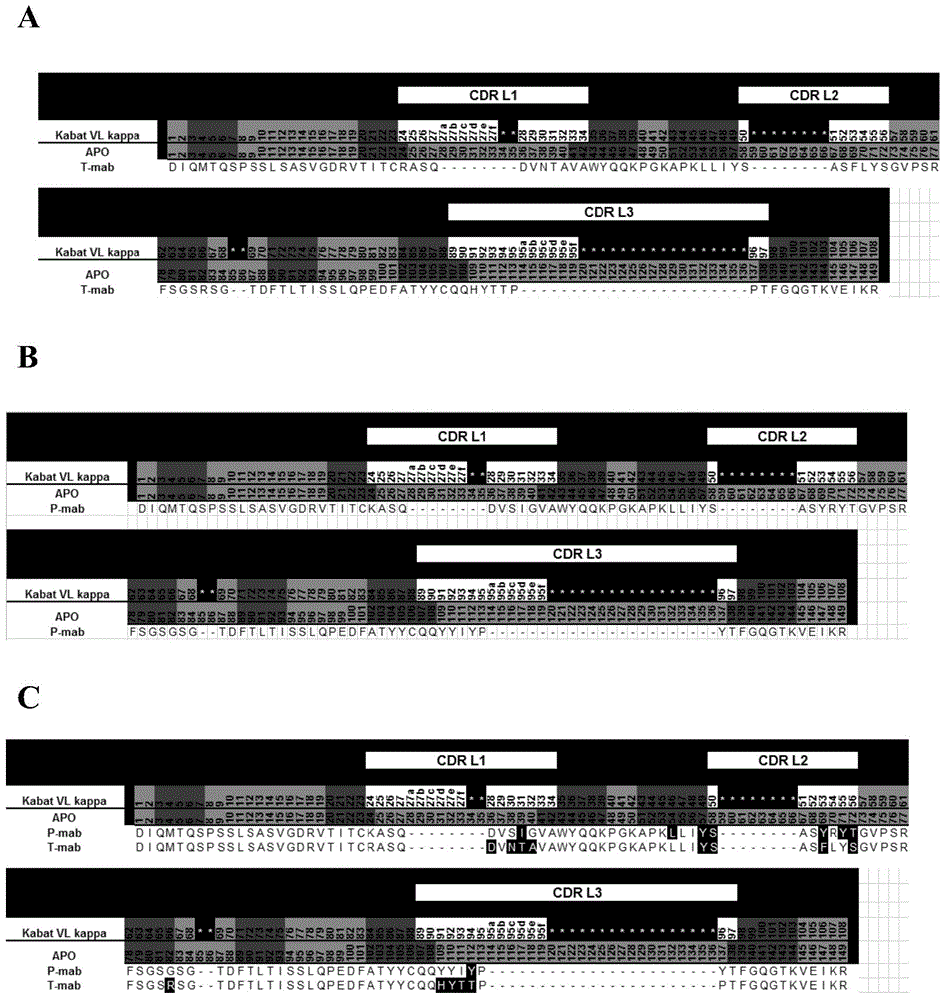
[0238] Example 4 Replace the original light chains of Tmab and Pmab with common light chains, and verify the effect of common light chains
[0239] 1. Construction of Tmab and Pmab monoclonal antibody eukaryotic expression vectors carrying a common light chain
[0240] Amino acid sequences of Trastuzumab and Pertuzumab full antibodies searched according to patent US2009 / 0285837A1 (patent in figure 2 and Figure 16 ), using the DNAworks online tool (http: / / helixweb.nih.gov / dnaworks / ) to design the corresponding coding DNA sequence, and obtain the heavy chain gene of Trastuzumab (SEQ ID NO: 16) and the heavy chain gene of Pertuzumab ( SEQ ID NO: 17). According to the amino acid sequence (SEQIDNO:1~6) of a group of common light chains obtained in Example 1, utilize DNAworks online tool (http: / / helixweb.nih.gov / dnaworks / ) to design the corresponding coding DNA sequence, and pass The common light chain genes CLC1 (SEQ ID NO: 7) and CLC5 (SEQ NO: 11) of the Pmab-Tmab bispecific ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com