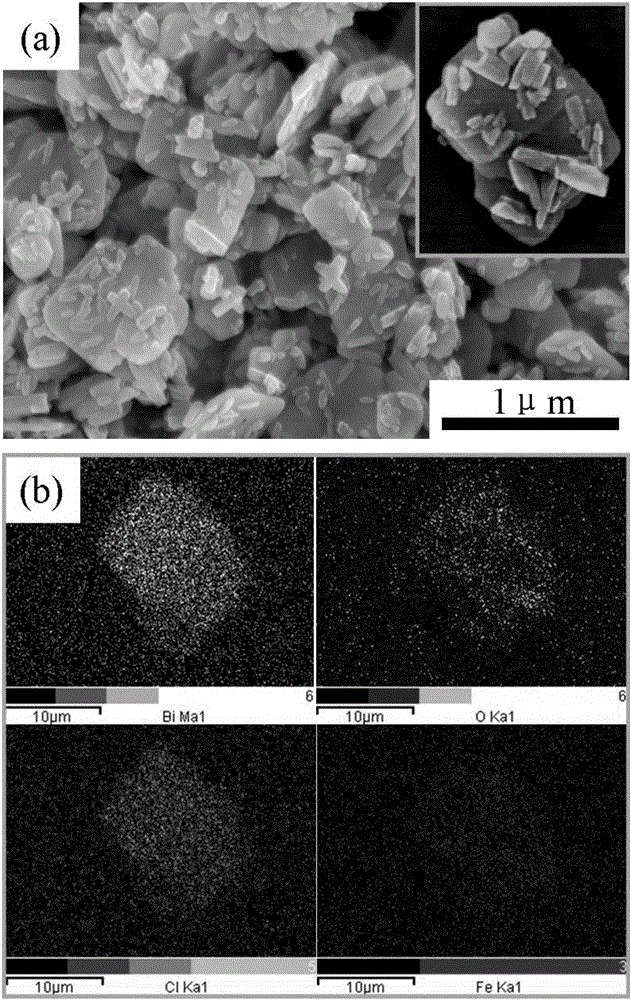
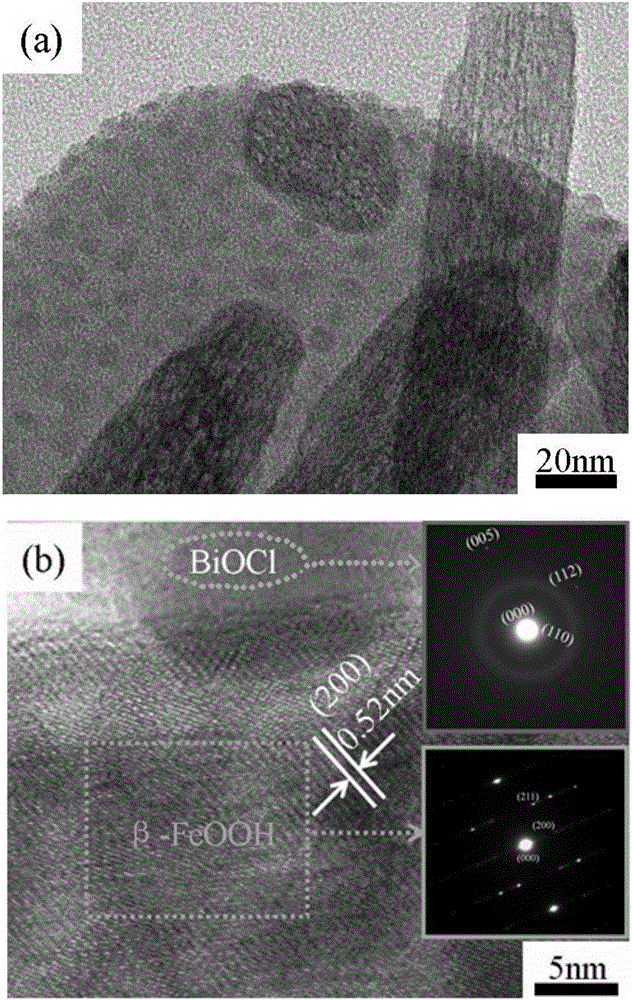
BiOCl/beta-FeOOH composite nanomaterial and preparation method thereof
A technology of composite nanomaterials and nanorods, applied in the field of BiOCl/β-FeOOH composite nanomaterials and its preparation, to achieve the effects of controllable product composition, less by-products, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] (1), the FeCl of 27g 3 ·6H 2 O was dissolved in 100 mL of distilled water, and magnetically stirred for 2 minutes at room temperature to fully dissolve it to obtain solution a.
[0022] (2), weigh 48.5g of Bi(NO 3 ) 3 ·5H 2 O was added to the above solution a, and magnetic stirring was continued for 1 minute to obtain solution b.
[0023] (3) Add 0.35 g of glucose into solution b, adjust the pH of the solution to 3 with HCl solution, and obtain solution c.
[0024] (4) Put the solution c into the reactor to carry out the hydrothermal synthesis reaction, the reaction time is 24 hours, and the reaction temperature is 100°C.
[0025] (5) After the reaction, the product was taken out, washed, centrifuged and dried to obtain the target product. The drying temperature was 70° C. and the drying time was 4 hours.
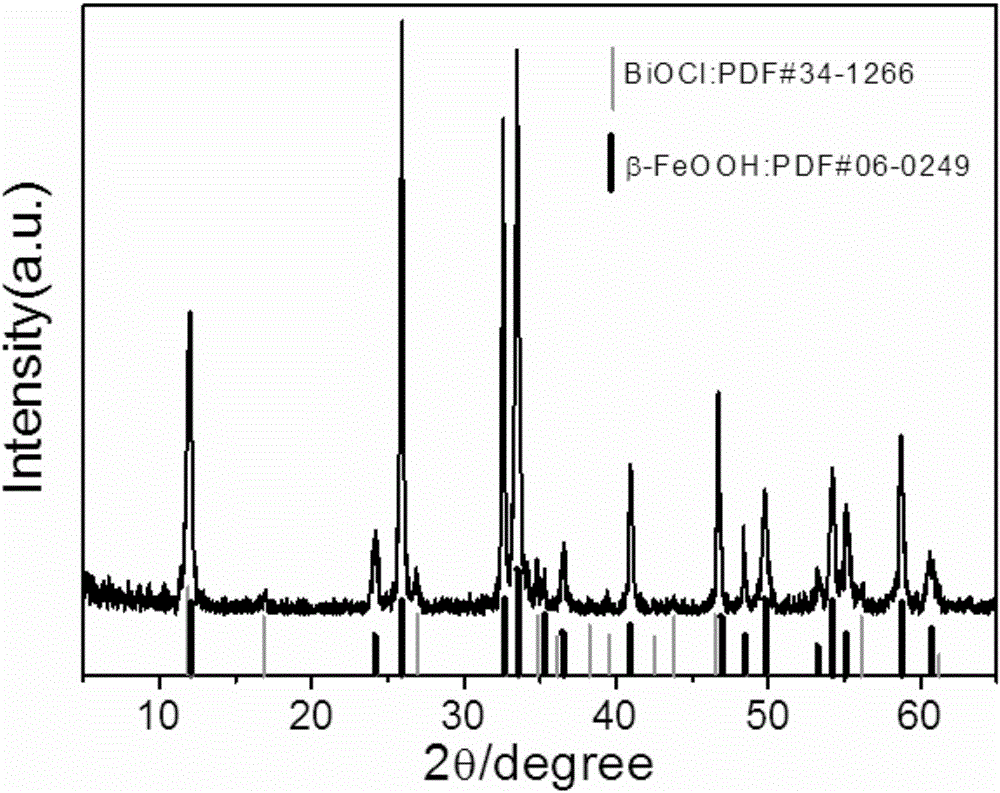
[0026] figure 1 It is the XRD spectrum pattern of the product obtained in Example 1. Comparing and analyzing all the XRD diffraction peaks of the product wit...
Embodiment 2
[0031] (1), the FeCl of 135g 3 ·6H 2 O was dissolved in 100 mL of distilled water, and magnetically stirred for 3 minutes at room temperature to fully dissolve it to obtain solution a.
[0032] (2), weigh 48.5g of Bi(NO 3 ) 3 ·5H 2 O was added to the above solution a, and magnetic stirring was continued for 1 minute to obtain solution b.
[0033] (3) Add 0.55 g of glucose into solution b, adjust the pH of the solution to 4 with HCl solution, and obtain solution c.
[0034] (4) Put the solution c into the reactor to carry out the hydrothermal synthesis reaction, the reaction time is 48 hours, and the reaction temperature is 80°C.
[0035] (5) After the reaction, the product was taken out, washed, centrifuged and dried to obtain the target product. The drying temperature was 80° C. and the drying time was 2 hours.
Embodiment 3
[0037] (1), the FeCl of 27g 3 ·6H 2 O was dissolved in 100 mL of distilled water, and magnetically stirred at room temperature for 1 minute to fully dissolve to obtain solution a.
[0038] (2), weigh 727.6g of Bi(NO 3 ) 3 ·5H 2 O was added to the above solution a, and magnetic stirring was continued for 3 minutes to obtain solution b.
[0039] (3) Add 0.85 g of glucose into solution b, adjust the pH of the solution to 6 with HCl solution, and obtain solution c.
[0040] (4) Put the solution c into the reactor to carry out the hydrothermal synthesis reaction, the reaction time is 36 hours, and the reaction temperature is 90°C.
[0041] (5) After the reaction, the product was taken out, washed, centrifuged and dried to obtain the target product. The drying temperature was 60° C. and the drying time was 6 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com