Synthesis method of tetraamminepalladium sulfate (II)
A technology of tetraammine palladium sulfate and tetraammine palladium dichloride, applied in chemical instruments and methods, ruthenium/rhodium/palladium/osmium/iridium/platinum compounds, inorganic chemistry, etc., can solve the problem of increased by-products and excessive sulfuric acid Many, increase costs and other issues, to achieve the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
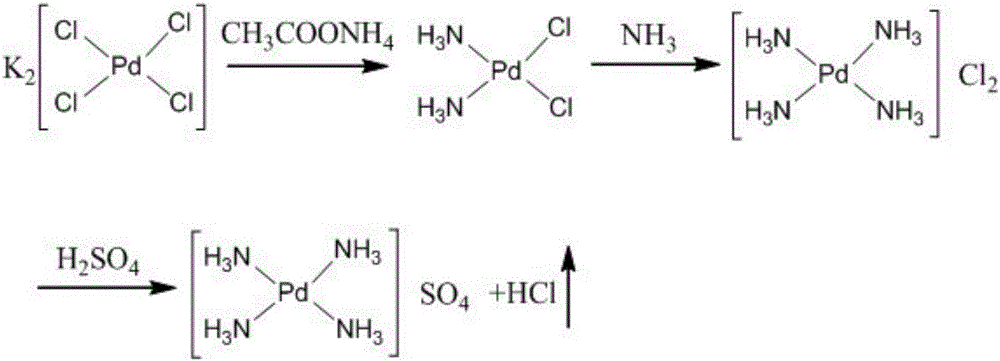
[0018] see figure 1 , in the embodiment of the present invention, respectively weigh 100 g of potassium chloropalladate and 55 g of ammonium acetate, dissolve in water and mix, add water to 6 L, adjust the pH value to 5 with acetic acid and reflux for 4 h, and filter the obtained solid after cooling, 62 g of cis-dichlorodiammine palladium (II) was obtained, with a yield of 96%.
[0019] Weigh 60g of cis-dichlorodiammine palladium (II), dissolve it with an appropriate amount of ammonia until the solution is transparent and clear, then concentrate the solution under reduced pressure to nearly dryness to obtain 68g of dichlorotetraammine palladium (II) solid, with a yield of 98 %.
[0020] Weigh 61.2g of dichlorotetraamminepalladium(II) and dissolve it in water, add 26.3mL of 10mol / L sulfuric acid solution, add water to 0.45L and then reflux for 5h, concentrate the solution to nearly dryness and then concentrate under reduced pressure to obtain 65.5g tetrasulfuric acid Ammonia ...
Embodiment 2
[0022] see figure 1 , in the embodiment of the present invention, respectively weigh 750 g of potassium chloropalladate and 450 g of ammonium acetate, dissolve in water and mix, add water to 38 L, adjust the pH value to 5 with acetic acid and reflux for 4 hours, and filter the obtained solid after cooling. 461 g of cis-dichlorodiammine palladium (II) was obtained, with a yield of 95%.
[0023] Take by weighing 460g cis-dichloro diammine palladium (II), dissolve it with an appropriate amount of ammonia until the solution is transparent and clear, then concentrate the solution under reduced pressure to near dryness to obtain 523g of dichloro tetraammine palladium (II) solid, with a yield of 98 %.
[0024] Weigh 490g of dichlorotetraamminepalladium (II) and dissolve it in water, add 220mL of 10mol / L sulfuric acid solution, add water to 4L and then reflux for 5h, concentrate the solution to near dryness and then concentrate under reduced pressure to obtain 518.4g of tetraamminepa...
Embodiment 3
[0026] see figure 1 , in the embodiment of the present invention, respectively weigh 1000 g of potassium chloropalladate and 600 g of ammonium acetate, dissolve them in water and mix them, add water to 55 L, adjust the pH value to 5 with acetic acid and reflux for 4 hours, and filter the obtained solid after cooling. 621 g of cis-dichlorodiammine palladium (II) was obtained, with a yield of 96%.
[0027] Take by weighing 620g cis-dichloro diammine palladium (II), dissolve it with an appropriate amount of ammonia until the solution is transparent and clear, then concentrate the solution under reduced pressure to near dryness to obtain 705g of dichloro tetraammine palladium (II) solid, with a yield of 98 %.
[0028] Weigh 612.5g of dichlorotetraamminepalladium(II) and dissolve it in water, add 263mL of 10mol / L sulfuric acid solution, add water to 5L and then reflux for 5h, concentrate the solution to nearly dryness and concentrate under reduced pressure to obtain 648g of tetraa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com