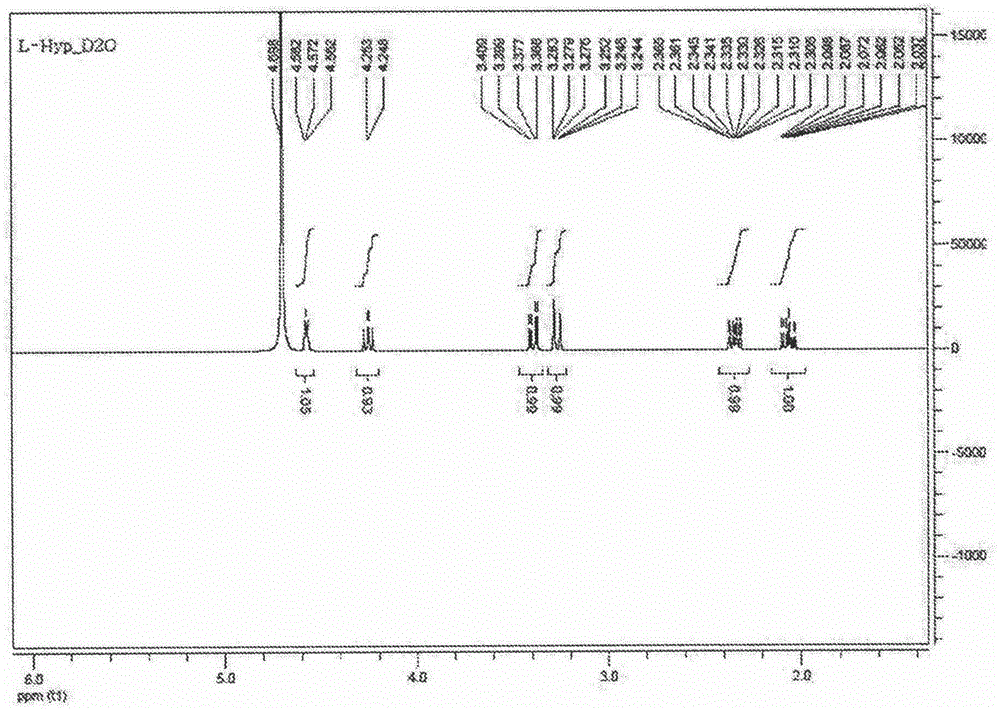
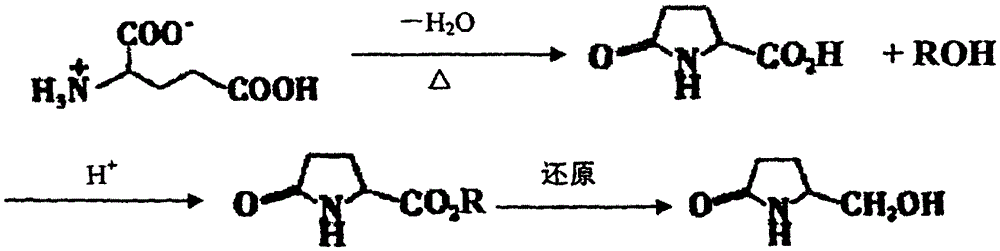
Preparation method for L-hydroxyproline
A technology of hydroxyproline and glutamic acid, which is applied in the field of preparation of L-hydroxyproline, can solve the problems of high production cost and slow production efficiency, and achieve low production cost, extended application range, yield and purity high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] This embodiment relates to a preparation method of L-hydroxyproline, specifically comprising the following steps:
[0038] Add 60kg of glutamic acid into a 200L electric heating stainless steel reaction tank, carry out oil bath dehydration reaction at 220-290°C, keep it at 140°C for 55min, and put it into 10L of water;
[0039] Stir and cool, centrifuge to obtain crude pyroglutamic acid, add water to the crude pyroglutamic acid at 65°C to saturation, centrifugally filter, cool to 35°C for crystallization, centrifuge the crystals, wash and dry to obtain pyroglutamic acid Fine product 28kg, yield 54% (based on glutamic acid feeding theoretical yield), melting point is 159~161 ℃, [a] 20 =-11.6 (C=2, H 2 O);
[0040] According to the feed ratio of pyroglutamic acid, absolute ethanol, and thionyl chloride as 1mol: 100ml: (0.06~1.2) mol, add 10L of absolute ethanol to a dry three-necked flask, cool to - Below 5°C, add 300ml of thionyl chloride dropwise, add 1000g of pyrogl...
Embodiment 2
[0048] This embodiment relates to a preparation method of L-hydroxyproline, specifically comprising the following steps:
[0049] Add 80kg of glutamic acid into a 200L electric heating stainless steel reaction tank, carry out dehydration reaction in oil bath at 250-270°C, keep it at 160°C for 50min, then put it into 10L of water;
[0050] Stir and cool, centrifuge to obtain the crude pyroglutamic acid, add water to the crude pyroglutamic acid at 50°C to saturation, centrifugally filter, cool to 30°C for crystallization, centrifuge the crystal, wash and dry to obtain pyroglutamic acid Fine product 37kg, yield 53.8% (based on glutamic acid feeding theoretical yield), melting point is 158.5 ~ 161 ° C, [a] 20 =-11.5 (C=2, H 2 O);
[0051] According to the feed ratio of pyroglutamic acid, absolute ethanol and concentrated sulfuric acid is 1mol: 100ml: (0.06 ~ 1.2) mol, add 10L of absolute ethanol to a dry three-necked flask, and cool down to -5°C with ice salt water Next, add 25...
Embodiment 3
[0059] This embodiment relates to a preparation method of L-hydroxyproline, specifically comprising the following steps:
[0060] Add 60kg of glutamic acid to a 200L electric heating stainless steel reaction tank, carry out oil bath dehydration reaction at 220-250°C, keep it at 140°C for 55min, and then put it into 10L of water;
[0061] Stir and cool, centrifuge to obtain crude pyroglutamic acid, add water to the crude pyroglutamic acid at 65°C to saturation, centrifugally filter, cool to 35°C for crystallization, centrifuge the crystals, wash and dry to obtain pyroglutamic acid Fine product 28kg, yield 54% (based on glutamic acid feeding theoretical yield), melting point is 158.5~161 ℃, [a] 20 =-11.5 (C=2, H 2 O);
[0062] According to the feed ratio of pyroglutamic acid, absolute ethanol, and hydrochloric acid is 1mol: 100ml: (0.06 ~ 1.2) mol, add 1L of absolute ethanol to a dry three-necked flask, and cool down to below -5°C with ice salt water , add 150g of hydrochlori...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com