Method for synthesizing 5-hydroxymethylfurfural ester derivative under enzymatic catalysis
A technology of hydroxymethylfurfural ester and hydroxymethylfurfural, which is applied in the field of bioenergy, fine chemical industry, and enzyme catalysis, can solve the problems of harsh reaction conditions, unfriendly use environment, and complicated process of chemical methods, and achieve repeated utilization, The effect of lowering the activation energy of the reaction and increasing the selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
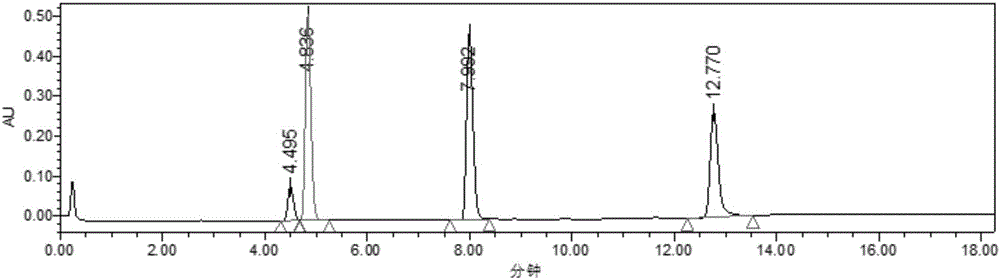
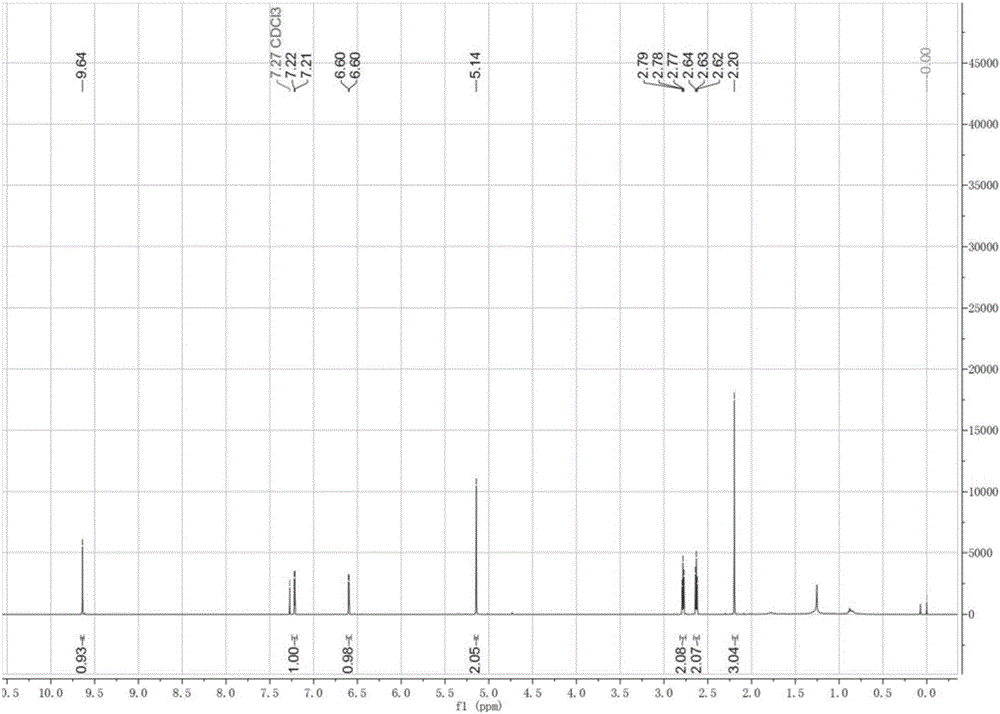
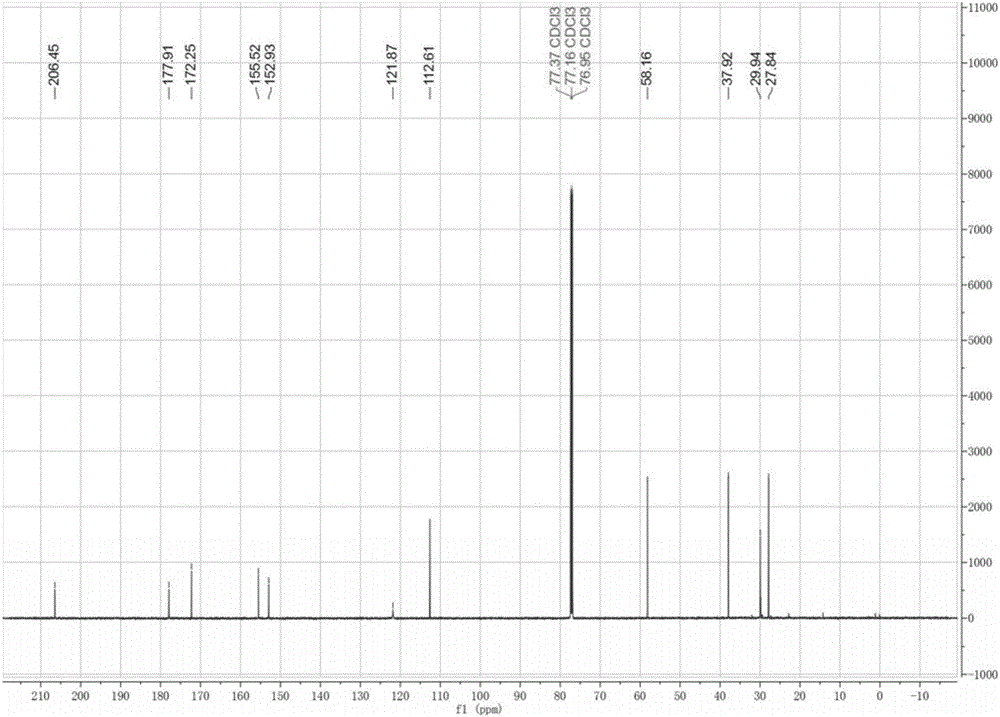
[0029] 1 mL of 2-methyltetrahydrofuran, 1 mL of levulinic acid, 50 mM HMF, 0.1 g Molecular sieves were added to a stoppered Erlenmeyer flask, then 20 mg of immobilized lipase B from Candida antarctica (purchased from Novozymes) was added, placed in a constant temperature oscillator at 40 °C and 200 r / min under normal pressure, and reacted for 12 h Finally, liquid phase analysis showed that the conversion rate of HMF reached 95%, and HMF levulinate was the only product. as attached figure 1 As shown, the retention times of levulinic acid, HMF, HMF levulinate and 9-fluorenone (internal standard) were 4.50, 4.84, 7.99 and 12.77 min, respectively. The target product was separated by column chromatography with a yield of 90%. NMR analysis (attached figure 2 and 3 ) shows that the target product is HMF levulinate.
Embodiment 2
[0031] 1 mL of 2-methyltetrahydrofuran, 1 mL of levulinic acid, 50 mM HMF, 0.1 g Molecular sieves were added to a stoppered Erlenmeyer flask, and then 20 mg of immobilized lipase from Thermomyces lanuginosus (purchased from Novozymes) was added, placed in a constant temperature oscillator at 40°C and 200 r / min under normal pressure, and reacted for 24 hours. , liquid phase analysis showed that the conversion of HMF was 3%, and HMF levulinate was the only product.
Embodiment 3
[0033] 1 mL of 2-methyltetrahydrofuran, 1 mL of levulinic acid, 50 mM HMF, 0.1 g Molecular sieves were added to a stoppered Erlenmeyer flask, then 20 mg of immobilized lipase derived from Pseudomonas cepacia (purchased from Japan Amano Company) was added, placed in a constant temperature oscillator at 40 ° C and 200 r / min under normal pressure, and after 24 hours of reaction, the liquid Phase analysis indicated 4% conversion of HMF with HMF levulinate as the only product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com