A core-shell SERS probe, its preparation method and its application in the detection of trace arsenate ions
A core-shell and probe technology, applied in the field of drinking water safety detection, achieves the effects of improved stability, quick and controllable assembly, and reduced detection limit
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] a) Take 10mL of citrate-stabilized Ag nanoparticles (prepared with reference to the document J.Phys.Chem.C, 2009, 113, 657., wherein the quality of the Ag nanoparticles is 1.1mg, and the diameter of the Ag nanoparticles is 40nm) at a rotating speed After being centrifuged under the condition of 6000rpm for 8 minutes, the lower layer of concentrate (volume is 0.2mL);
[0027] b) Disperse the concentrated solution obtained in step a) into 3 mL of ethylene glycol solution, and add 1 g of sodium acetate and 0.02 g of ferric nitrate in sequence, and then stir vigorously to completely dissolve the reactants;
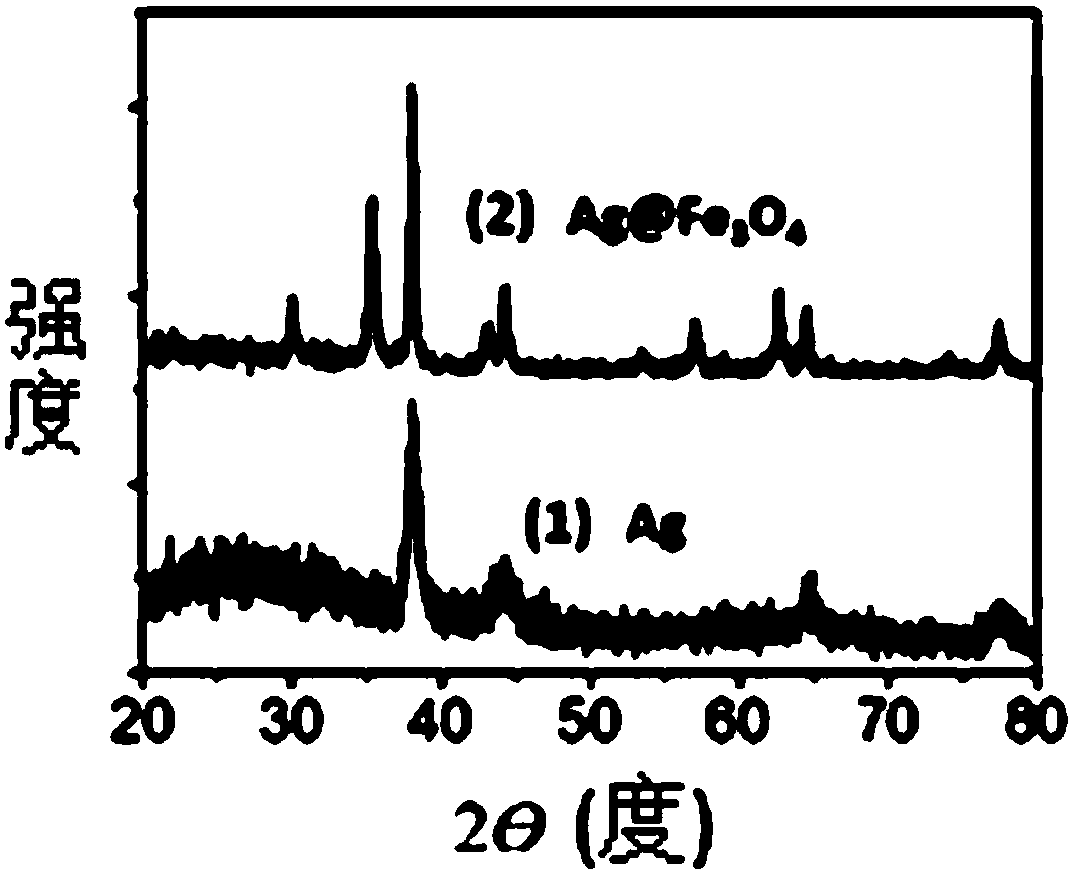
[0028] c) Put the mixture obtained in step b) in a reactor, and conduct a hydrothermal reaction at 200°C for 10 hours to obtain Ag@Fe 3 o 4 Colloidal solution of core-shell nanoparticles, the product quality is 3.3 mg.
[0029] Embodiment 1 performance test
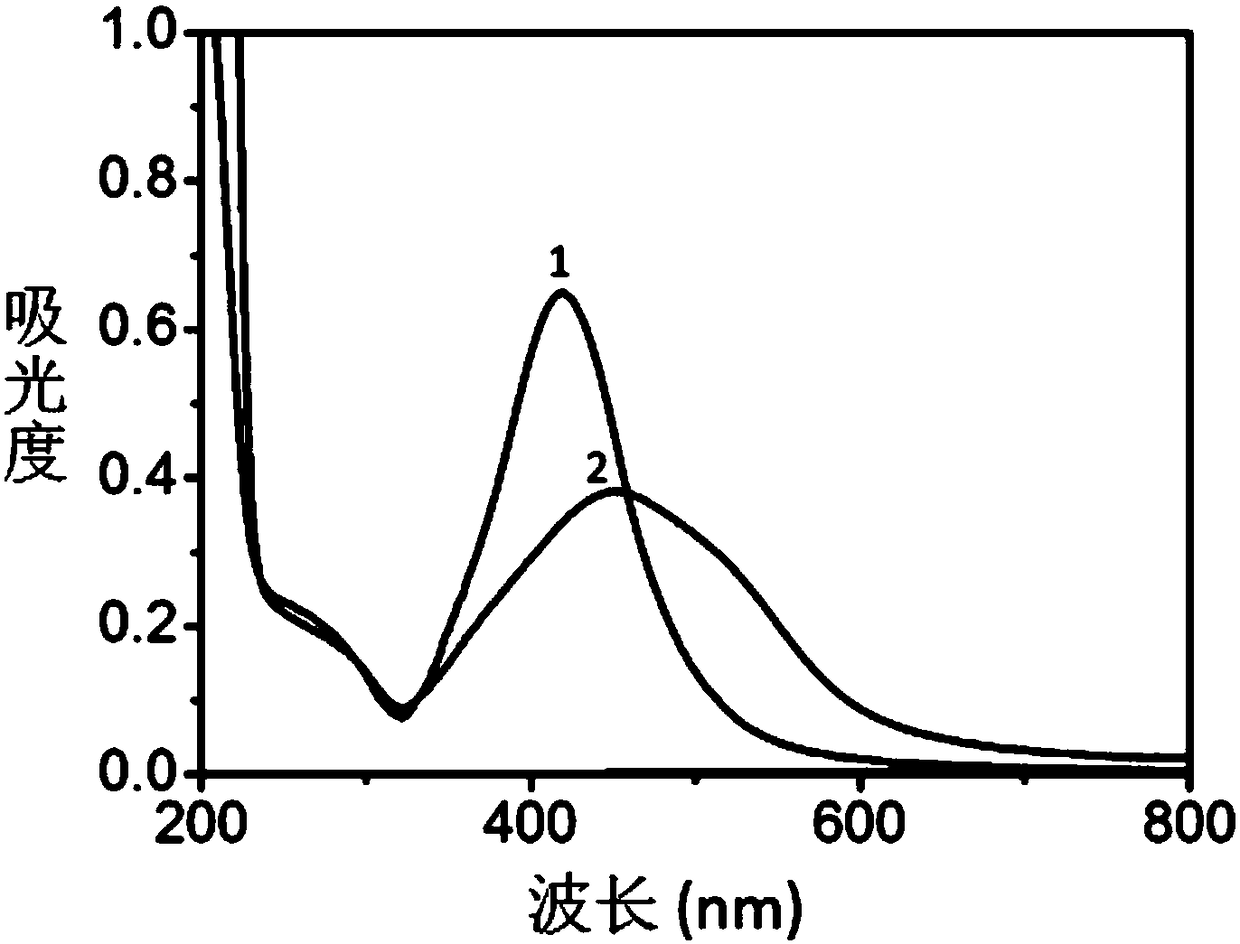
[0030] a) 2mg Ag@Fe 3 o 4 The core-shell nanoparticles were dispersed in 2 mL of sodium arsenate aqueous so...
Embodiment 2
[0037] a) get 10mL of citrate-stabilized Ag nanoparticles (wherein the quality of Ag nanoparticles is 1.1mg, and the diameter of Ag nanoparticles is 60nm) after centrifugation under the condition of 5000rpm for 6 minutes, take the lower layer concentrated solution (volume is 0.2mL);
[0038] b) Disperse the concentrated solution obtained in step a) into 3 mL of ethylene glycol solution, and add 1 g of sodium acetate and 0.02 g of ferric nitrate in sequence, and then stir vigorously to completely dissolve the reactants;
[0039] c) Put the mixture obtained in step b) in a reactor, and conduct a hydrothermal reaction at 200°C for 10 hours to obtain Ag@Fe 3 o 4 Colloidal solution of core-shell nanoparticles, the product quality is 4.1 mg.
[0040] Ag@Fe 3 o 4 The core-shell SERS probe has a diameter of 80nm; the Ag core has a diameter of 60nm, Fe 3 o 4 The thickness of the shell layer is 10 nm.
[0041] attached Figure 5 yes get Ag@Fe 3 o 4 Core-shell SERS probe detect...
Embodiment 3
[0043] a) Get 10mL of citrate-stabilized Ag nanoparticles (wherein the quality of Ag nanoparticles is 1.1mg, and the diameter of Ag nanoparticles is 80nm) after centrifugation under the condition of 3000rpm for 5 minutes, take the lower layer concentrated solution (volume is 0.2mL);
[0044] b) Disperse the concentrated solution obtained in step a) into 3 mL of ethylene glycol solution, and add 1 g of sodium acetate and 0.02 g of ferric nitrate in sequence, and then stir vigorously to completely dissolve the reactants;
[0045] c) Put the mixture obtained in step b) in a reactor, and conduct a hydrothermal reaction at 200°C for 10 hours to obtain Ag@Fe 3 o 4 Colloidal solution of core-shell nanoparticles, the product quality is 4.6mg.
[0046] Ag@Fe 3 o 4 The core-shell SERS probe has a diameter of 100nm; the Ag core has a diameter of 80nm, Fe 3 o 4 The thickness of the shell layer is 10 nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com