Paclitaxel palmitate liposome and preparation method thereof
A technology of palmitate and paclitaxel, applied in the field of medicine, can solve the problems of liver and kidney function and reproductive function damage, poor compatibility of infusion equipment, frequent allergic reactions, etc., so as to overcome the poor drugability, improve antitumor properties, and improve drug concentration. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
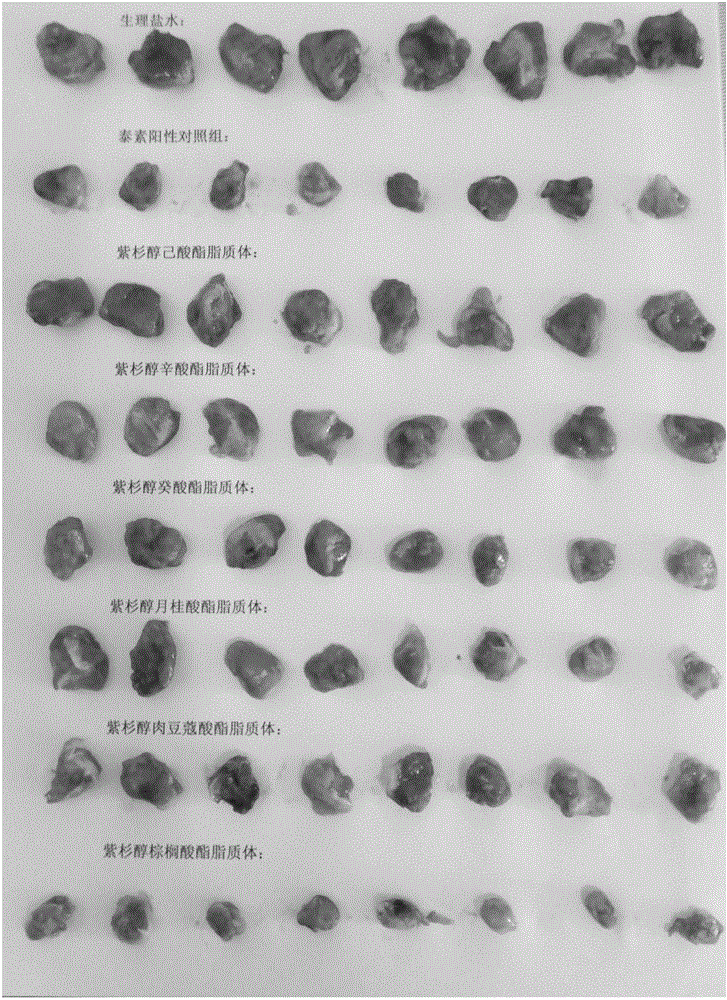
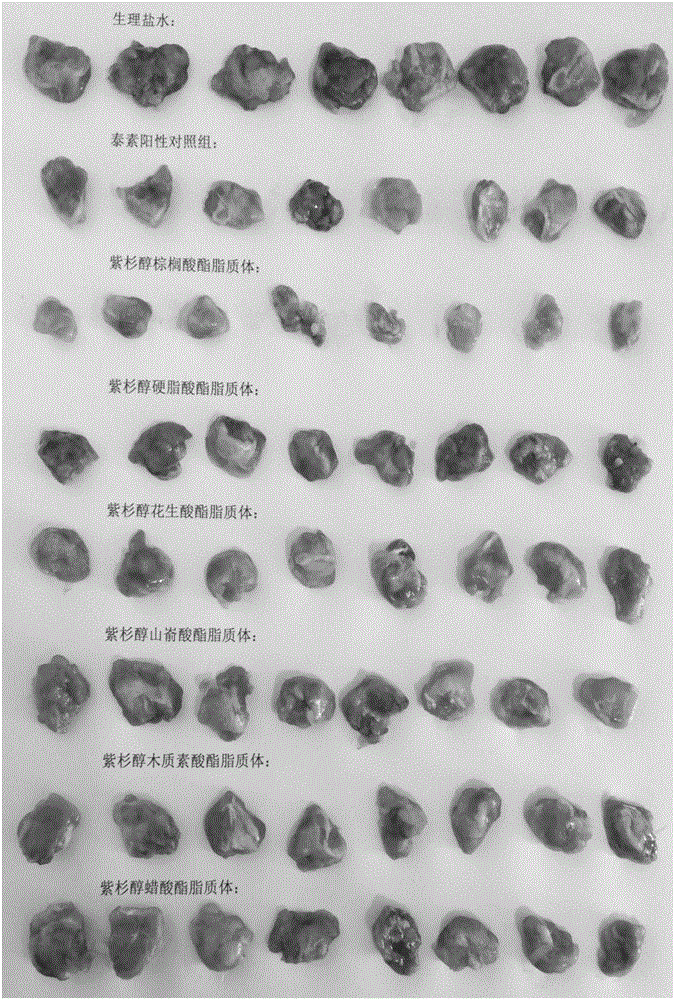
[0063] Example 1: Comparative evaluation of series of different paclitaxel fatty acid esters in vivo efficacy tests
[0064] Chinese patent CN1202166A and literature (Shaukat Ali, Imran Ahmad etc., Hydrolyzablehydrophobic taxanes: synthesis and anti-cancer activities, Anti-Cancer Drugs, 2001, 12:117-128; Walter R.Perkins, Imran Ahmad etc., Novel therapeutic nano-resicles ( ): trapping poorly water soluble compounds, International Journal of Pharmaceutics 2000, 200:27–39), which records a kind of α-bromo-based fatty acid ester of paclitaxel, and the carbon chain length of the fatty acid covered is paclitaxel between C6-C16 Fatty acid ester prodrug; U.S. patent (US 7,235,583B1) and international patent (WO 00 / 53231), have studied a paclitaxel fatty acid ester and its preparation, and its fatty acid length is C8-C26. The above-mentioned patent documents have recorded paclitaxel fatty acid ester prodrugs with fatty acid carbon chain lengths between C6-C26, but no differential rese...
Embodiment 2
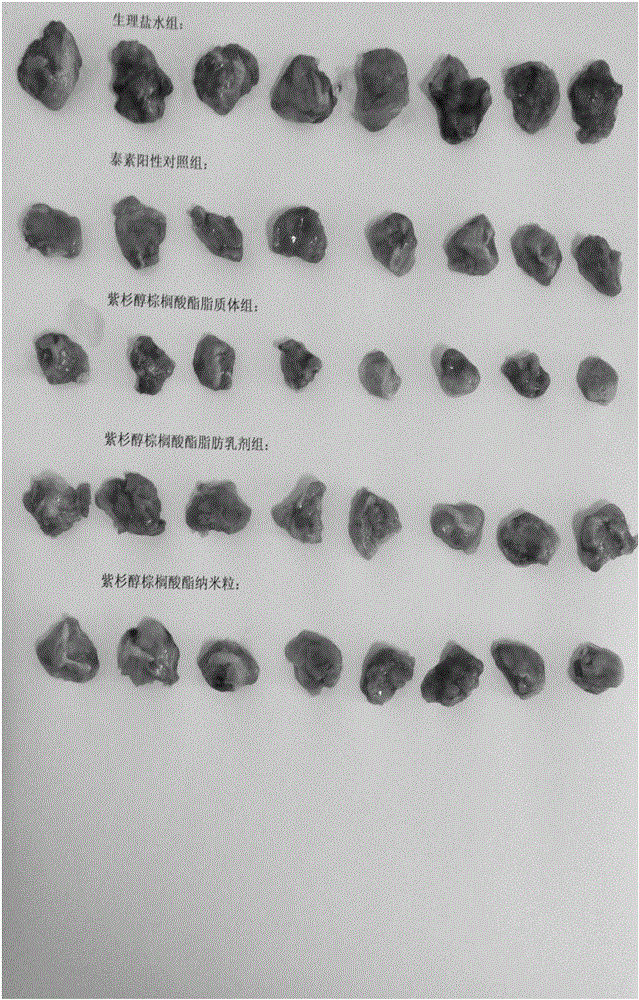
[0085] Example 2: Study on the druggability of paclitaxel palmitate in different formulations
[0086] The druggability and antitumor effect of liposomes, polymer micelles, fat emulsions, and nanoparticles were compared and studied respectively. For the sake of conservatism, the fixed drug loading was 3 mg / ml for parallel comparison. With reference to the current clinical dosage of paclitaxel ( The total dosage for adults is about 400mg each time), and the drug loading of 3mg / ml is only a lower requirement. On this basis, research on related preparations is carried out, and the main research programs and results are summarized as follows:
[0087] 1. Study on the druggability of preparations
[0088] 1.1 Liposome
[0089] Weigh 300mg of paclitaxel palmitate, 3.5g of high-purity egg yolk lecithin (EPCS), 0.3g of DSPE-PEG2000, add 3g of propylene glycol, heat and dissolve at 65°C to obtain an organic phase; weigh 85g of water for injection, heat to 65°C, Stir and dissolve to ...
Embodiment 3
[0113] Example 3: Importance of Propylene Glycol Infusion for Paclitaxel Palmitate Liposome Development
[0114] Commonly used liposome preparation methods include thin film evaporation, reverse evaporation, and ethanol injection. Among them, thin film evaporation has poor controllability and cumbersome steps in large-scale production. Therefore, we plan to use relatively simple reverse evaporation and ethanol injection. Injection method to prepare liposomes. But for paclitaxel palmitate, reverse evaporation method and ethanol injection method all can't realize the preparation of this liposome well, when selecting propylene glycol to inject, just received fundamental effect. In Example 2 above, good liposomes can be prepared by injecting a small amount of propylene glycol, so taking this as an example, the commonly used reverse evaporation method and ethanol injection method are respectively verified using the same prescription. The methods and results are as follows:
[011...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com