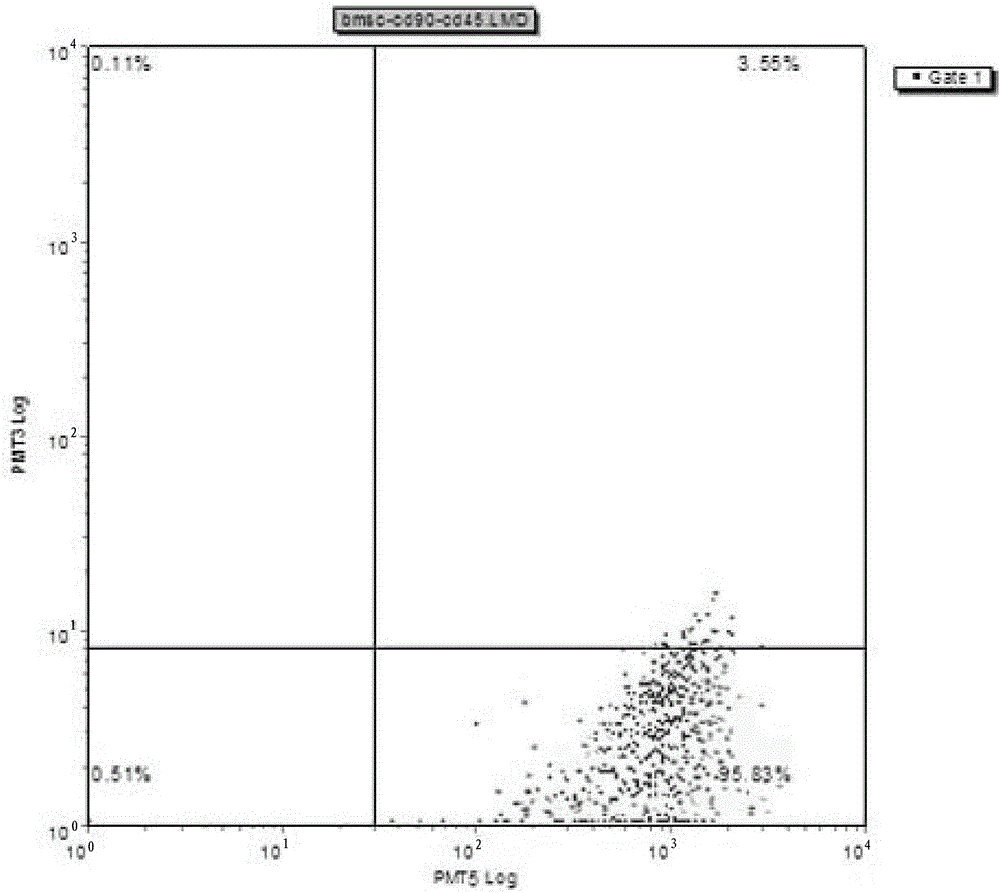
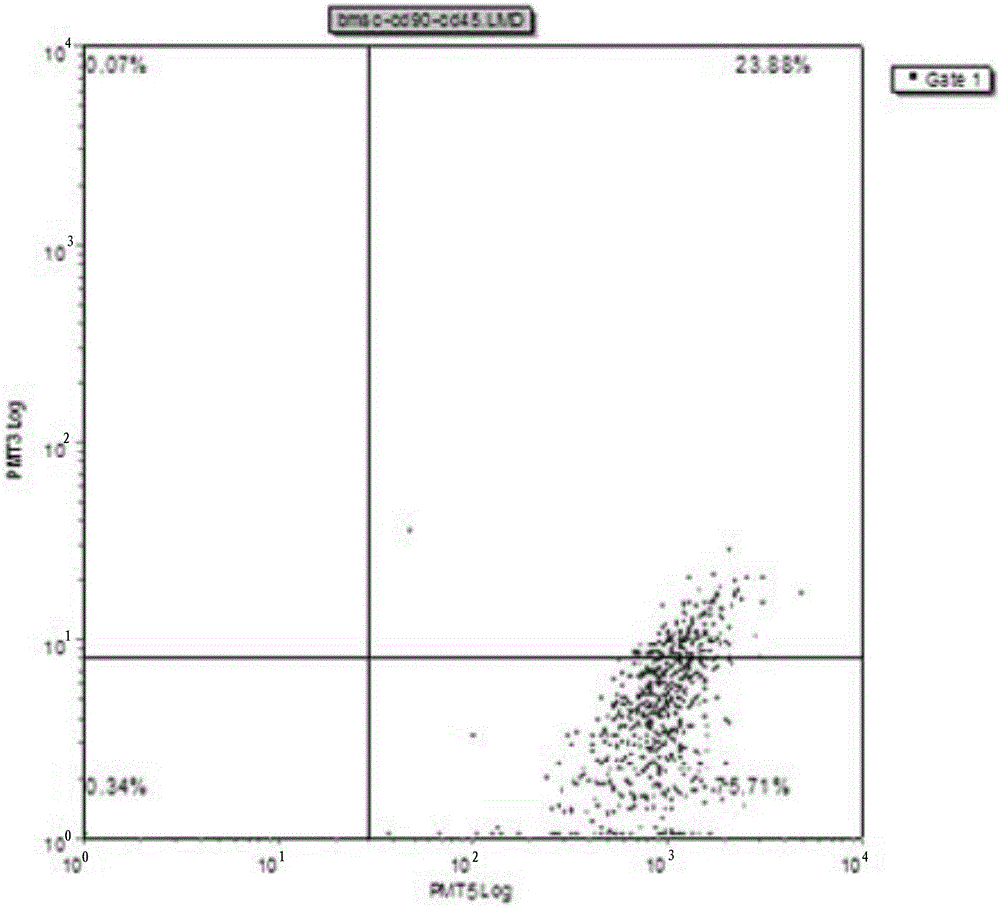
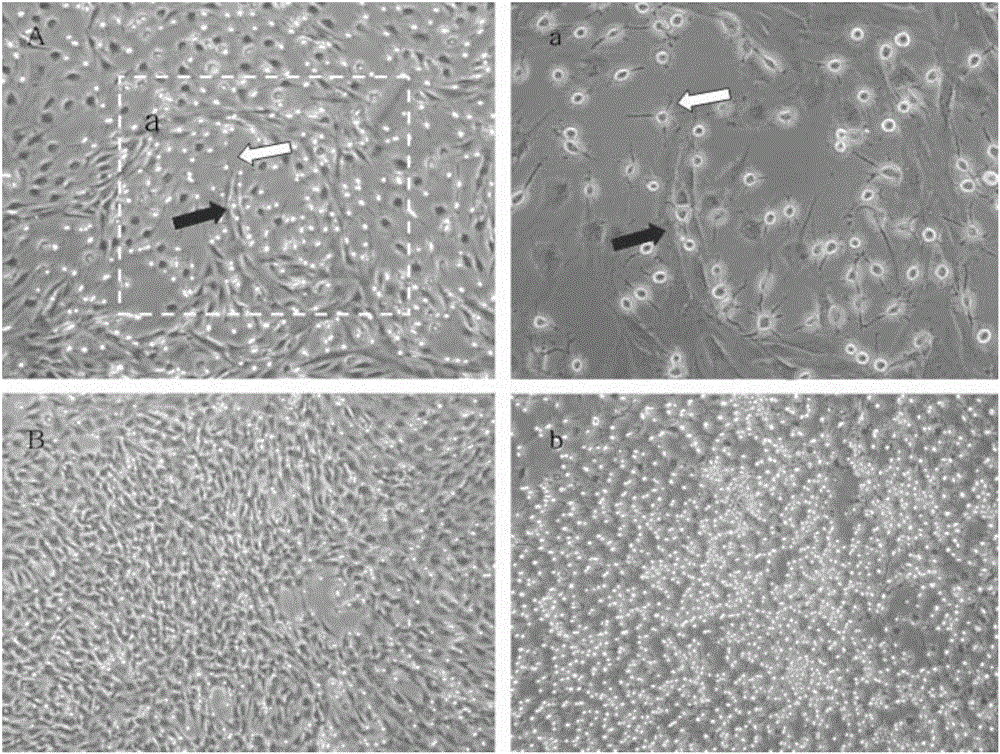
Method for rapidly obtaining and purifying bone marrow stroma stem cells
A technology for bone marrow stromal cells and stem cells, which is applied in the field of rapid acquisition and purification of bone marrow stromal stem cells, and can solve the problems of the purity and speed of BMSCs not meeting the requirements.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0029] The substantive content of the present invention will be further described below in conjunction with the implementation of the present invention, but the present invention is not limited thereto. The materials used in the present invention, unless otherwise specified, are commercially available products.
[0030] Table 1 shows the CD antigens on the surface of stem cells. You can choose a CD antigen on the surface of stem cells and a CD antigen on the surface of non-stem cells to form a screening marker:
[0031]
[0032] 1. Preparation of bone marrow stromal cell suspension
[0033] 1) Prepare serum-free medium α-MEM
[0034] Take 10.2g α-MEM (Minimum Essential Medium Alpha), 2.2g NaHCO3 and 100U heparin, dilute to 1L with deionized water, and use the obtained solution for later use.
[0035] 2) Method for resuspending bone marrow stromal cells
[0036] Use a syringe to extract α-MEM with a heparin concentration of 100U / L and blow repeatedly to obtain the origina...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com