Preparation method of chromium trichloride
A technology of chromium chloride and chromium hydroxide, applied in the field of chemistry, can solve the problems of low yield, high production cost, unenvironmental protection, etc., and achieve the effects of improving output and yield, low cost and reducing pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The preparation of embodiment 1 chromium chloride
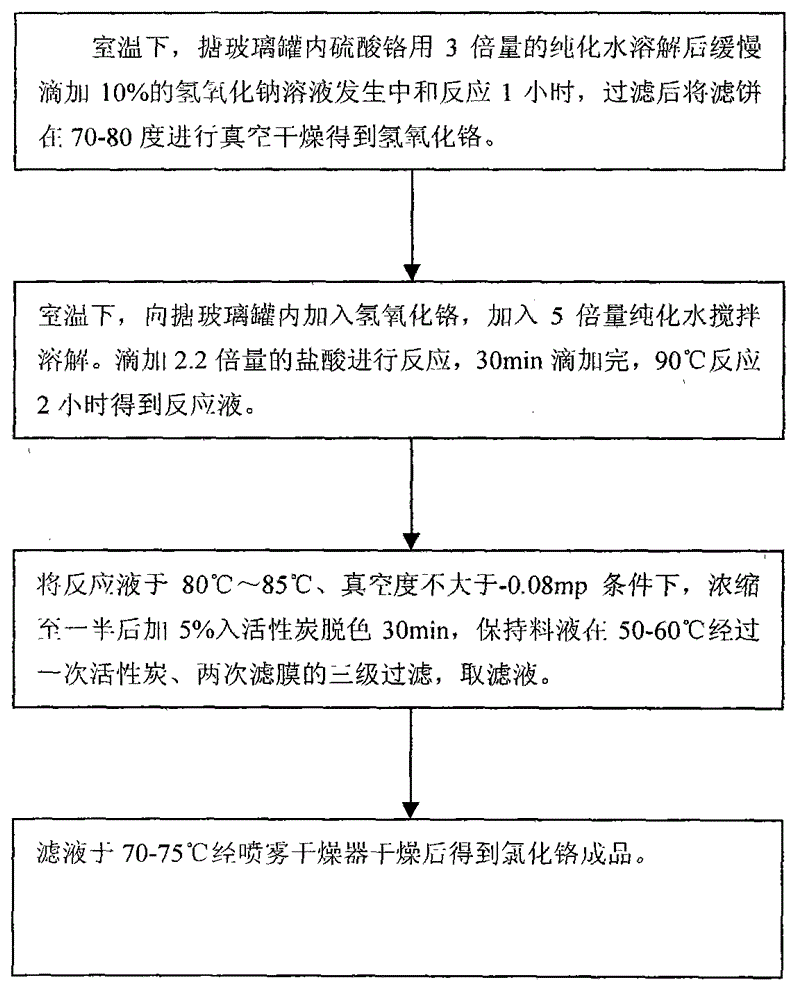
[0029] 1) At room temperature, add 50kg of chromium sulfate and 150kg of purified water into the glass-lined tank, stir to dissolve. Slowly add 320L of 8% sodium hydroxide solution dropwise to undergo a neutralization reaction for 1 hour. After filtering, vacuum-dry the filter cake at 70-80°C to obtain 15.45kg of chromium hydroxide.
[0030] 2) At room temperature, add 15.45kg of chromium hydroxide obtained in the previous step into the glass-lined tank, add 77.25kg of purified water and stir to dissolve. 30.9 kg of concentrated hydrochloric acid was added dropwise for reaction, and the dropwise addition was completed in 30 minutes, and reacted at 90° C. for 2 hours to obtain 112 L of reaction solution.
[0031] 3) Concentrate the reaction solution to 56L at 80°C to 85°C with a vacuum degree not greater than -0.08mp, add 0.75kg into activated carbon for decolorization for 30 minutes, and keep the feed liquid at 50-60°...
Embodiment 2
[0033] The preparation (best scheme) of embodiment 2 chromium chlorides
[0034] 1) At room temperature, add 50kg of chromium sulfate and 150kg of purified water into the glass-lined tank, stir to dissolve. Slowly add 250 L of 10% sodium hydroxide solution dropwise to undergo a neutralization reaction for 1 hour, and after filtration, vacuum-dry the filter cake at 70-80 degrees to obtain 16.15 kg of chromium hydroxide.
[0035] 2) At room temperature, add 16.15kg of chromium hydroxide obtained in the previous step into the glass-lined tank, add 80.75kg of purified water and stir to dissolve. 32.3 kg of concentrated hydrochloric acid was added dropwise for reaction, and the dropwise addition was completed in 30 minutes, and reacted at 90° C. for 2 hours to obtain 118 L of reaction solution.
[0036] 3) Concentrate the reaction solution to 59L at 80°C to 85°C and the vacuum degree is not greater than -0.08mp, then add 0.8kg into activated carbon for decolorization for 30min, ke...
Embodiment 3
[0038] The preparation of embodiment 3 chromium chlorides
[0039] 1) At room temperature, add 50kg of chromium sulfate and 150kg of purified water into the glass-lined tank, stir to dissolve. Slowly add 166.7L of 15% sodium hydroxide solution dropwise to undergo a neutralization reaction for 1 hour. After filtration, vacuum-dry the filter cake at 70-80°C to obtain 16.10kg of chromium hydroxide.
[0040] 2) At room temperature, add 16.10 kg of chromium hydroxide obtained in the previous step into the glass-lined tank, add 80.5 kg of purified water and stir to dissolve. 32.2 kg of concentrated hydrochloric acid was added dropwise for reaction, and the dropwise addition was completed in 30 minutes, and reacted at 90° C. for 2 hours to obtain 115 L of reaction solution.
[0041] 3) Concentrate the reaction solution to 55.5L at 80°C to 85°C and the vacuum degree is not greater than -0.08mp, add 0.8kg into activated carbon for decolorization for 30min, keep the feed liquid at 50-6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com