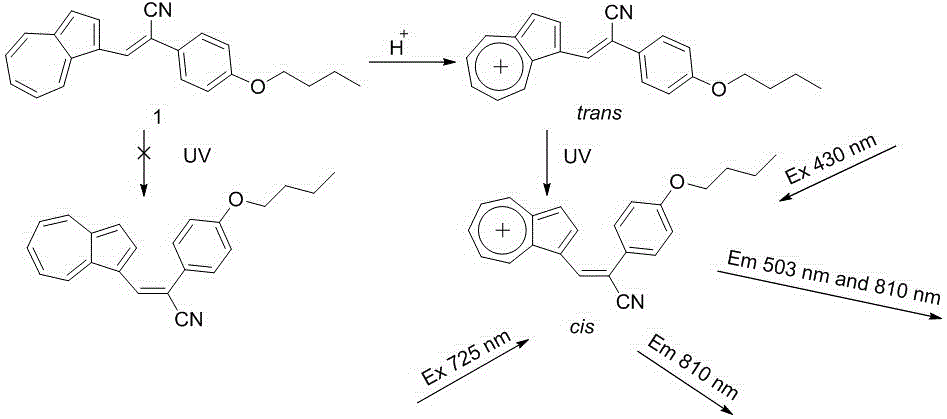
Nitrile-group-containing azulene-styrene derivatives adopted as near infrared fluorescence probe as well as preparation method and application of nitrile-group-containing azulene-styrene derivative
A technology of styrene derivatives and fluorescent probes, applied in the field of fluorescent dyes, to achieve the effect of solving the single response of the probe and simple synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] (1) Synthesis of compound 3
[0049]Add 1mL of N,N-dimethylformamide to a 50mL round bottom flask, slowly add 0.1mL of phosphorus oxychloride at 0 ℃, then slowly rise to room temperature and react for 30 minutes, then add azulene (0.128 mg, 1 mmol) , react at room temperature for 1 hour, add dilute sodium hydroxide solution to adjust the pH to neutral, extract with dichloromethane three times (15mL×3), combine the organic layers, wash with saturated brine, and dry over anhydrous sodium sulfate. After vacuum concentration, separation and purification by column chromatography using petroleum ether: ethyl acetate = 10:1 (v / v) as the eluent gave dark purple oily liquid 3 with a yield of 95%. 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 10.38 (s, 1H), 9.62 (d, J = 9.6 Hz, 1H), 8.54 (d, J = 9.7 Hz, 1H), 8.30 (d, J = 4.2 Hz, 1H), 7.89 (t, J = 9.7 Hz, 2H), 7.66 (t, J = 9.9 Hz, 1H), 7.56 (t, J = 9.7 Hz, 2H), 7.36 (d, J = 4.1 Hz, 1H).
[0050] (2) Synthesis of Compound 4...
Embodiment 2
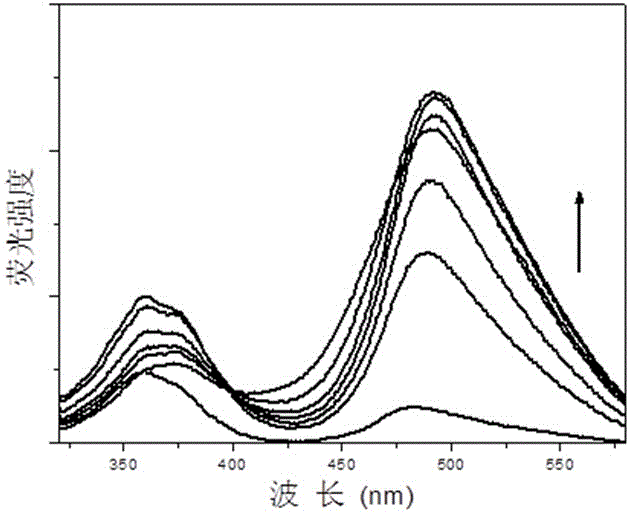
[0057] Compound I obtained above was dissolved in chloroform to form a 0.5 μmol / L solution. Add 2mL of the solution into a 1cm×1cm×4cm stoppered cuvette, then add different volumes of trifluoroacetic acid with a micro-syringe and mix evenly for 1 minute, then test its fluorescence emission spectrum, λ ex = 302 nm, the result is as figure 2 shown. With the continuous addition of trifluoroacetic acid, the fluorescence intensity of the solution gradually increased.
Embodiment 3
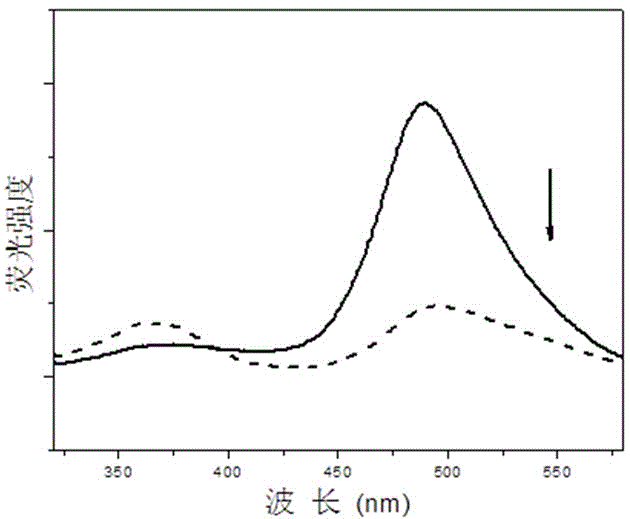
[0059] Compound I obtained above was dissolved in chloroform to form a 0.5 μmol / L solution. Add 2mL of the solution into a 1cm×1cm×4cm stoppered cuvette, then add a certain volume of trifluoroacetic acid with a micro-syringe and mix evenly for 1 minute, then place the solution after adding the acid under 365 nm light Under 30 minutes, test its fluorescence emission spectrum, λ ex =302 nm, with the increase of illumination time, the color of the solution will change significantly, from yellow-green to dark green, and the fluorescence intensity will also decrease obviously, the results are as follows image 3 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com