Preparation method and antineoplastic activity of novel Ru(II) complex containing 4-nitrobenzene
A technology of anti-tumor activity and complexes, applied in the field of anti-tumor activity, can solve the problems of low water solubility, high toxicity and side effects, drug resistance of cancer cells, etc., and achieve the effect of enhanced binding ability and high affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
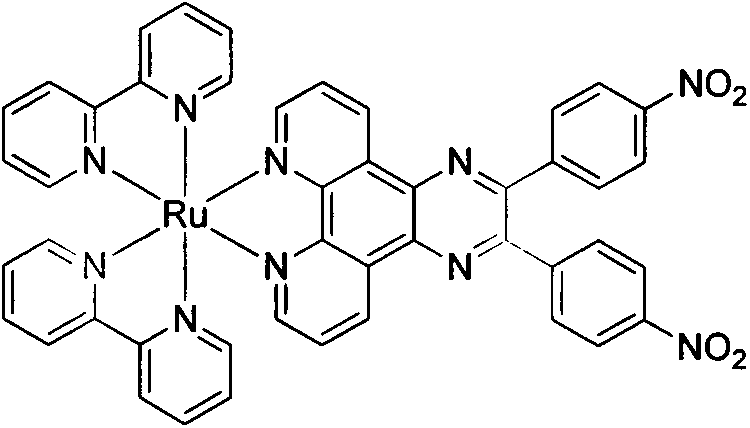
[0020] A kind of synthesis that contains 2,3-two (4-nitrophenyl) pyrazine [2,3-f] [1,10] phenanthroline novel Ru (II) complexes, comprises the following process steps:
[0021] (1) Synthesis of 5,6-diketone-1,10-phenanthroline
[0022] Add 10g1,10-phenanthroline (55.6mmol) in a 250mL three-necked flask, quickly add 60mL of concentrated sulfuric acid under ice-salt bath conditions, quickly add 13.2g KBr (111mmol) after dissolving, slowly add 30mL of concentrated nitric acid dropwise, and heat up to React at 110°C for 2.5 hours. After the reaction is completed, cool to room temperature. Pour the reaction solution into 500 g of crushed ice and neutralize it with 10 mol / L NaOH solution. A large amount of yellow solid precipitates, which is filtered by suction, washed with a large amount of distilled water, and recrystallized from ethanol to obtain a yellow color. Needle crystals were suction filtered and dried in vacuo to obtain 7.17 g of solid, with a yield of 60.8%. The reactio...
Embodiment example 2
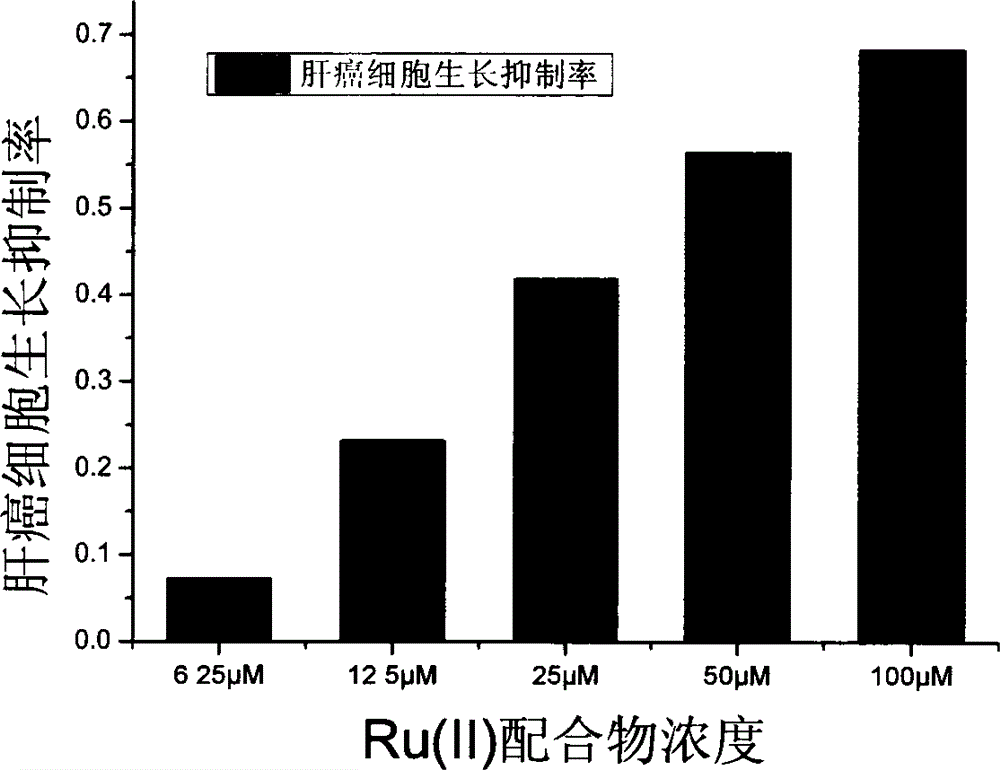
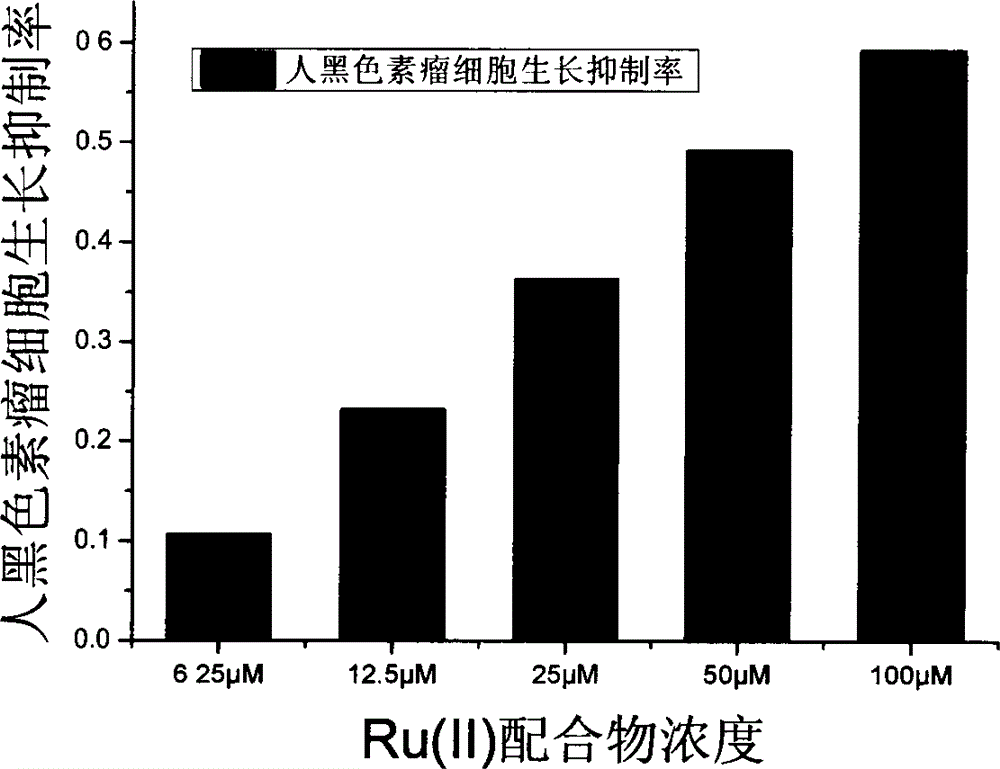
[0036] Implementation Case 2: Anti-tumor activity experiment of Ru(II) complexes
[0037]The invention adopts the MTT method to measure the toxicity of Ru(II) complexes on tumor cells in vitro, and takes liver cancer cell line Hep G2 and human melanoma cell line F10-B16 as detection objects. The cultured cancer cell suspension was inoculated into a clean 96-well culture plate, 200 μL / well, and the obtained Ru(II) complex was added to the cell culture plate in a concentration gradient (6.25 μM-100 μM), and each Well 100 μL, the concentration of each Ru(II) complex is 2 parallel plates, no Ru(II) complex is added to the blank control group, only the culture solution is added. Place in a 37°C incubator at a constant temperature for 48 hours, add 20 μL of 5 g / L MTT to each well, continue to incubate for 5 hours, then add 100 μL of SDS to each well, and measure the absorbance value of each well at a wavelength of 570 nm on a microplate reader after 10 hours. The inhibition rate wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com