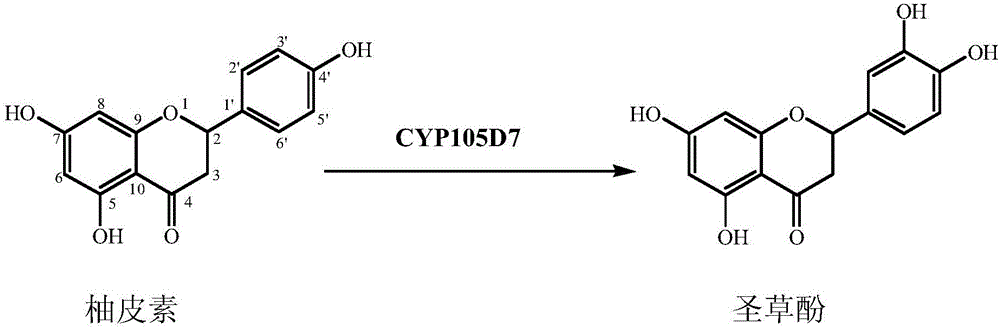
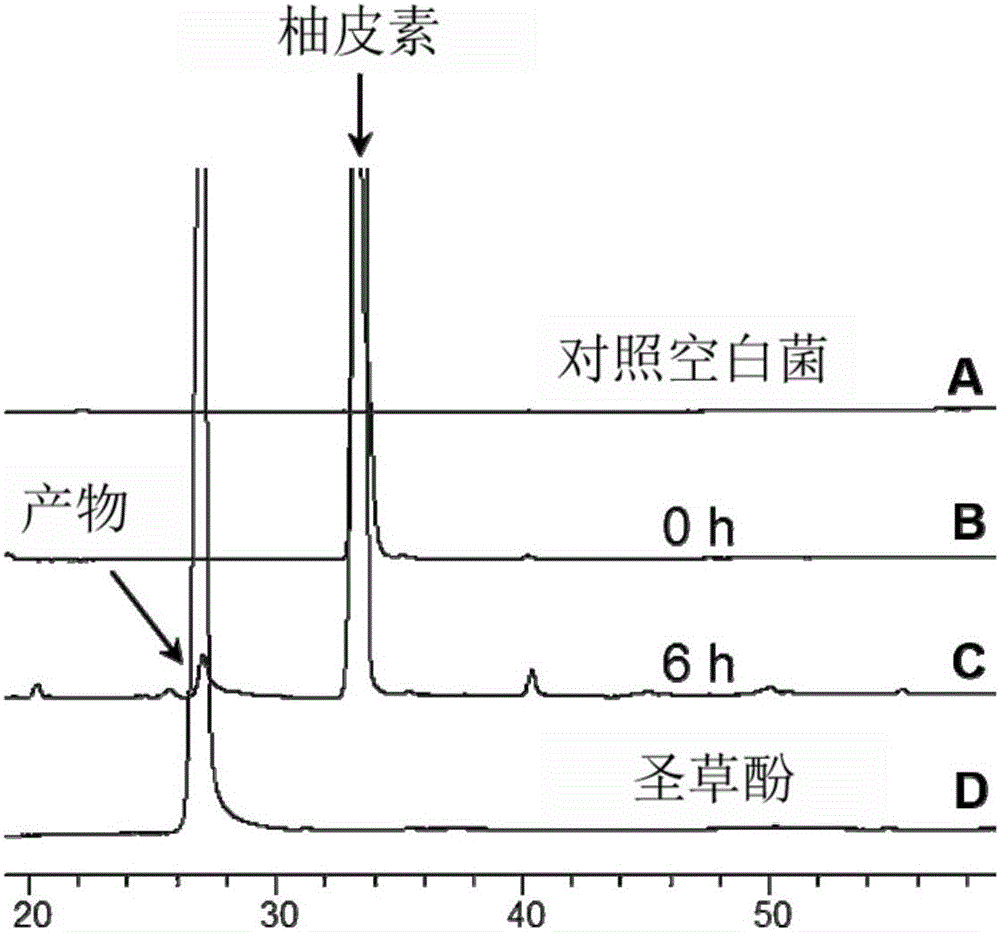
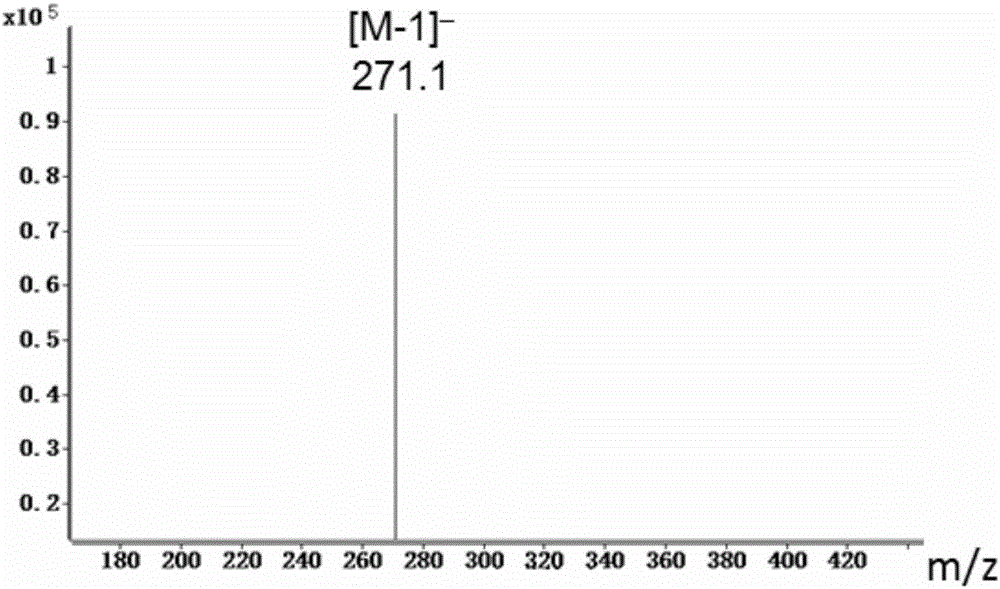
Method for obtaining eriodictyol by biologically transforming naringenin
A biotransformation and naringenin technology, applied in the biological field, can solve the problems of no hydroxylation, chemical structure and increased difficulty in chemically synthesizing flavonoids, and achieve the effects of easy separation, reduction of side reaction products, and simple operation procedures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0020] Such as figure 1 As shown, the method for biotransforming naringenin of the present invention to obtain eriodictyol comprises the following steps:
[0021] 1. Obtain co-expression recombinant Escherichia coli
[0022] The sav7469 gene encoding CYP105D7 of Streptomyces avermitilis MA4680 (its gene sequence is shown in SEQID NO.1) is from CL_228_E11 cosmid (http: / / avermitilis.ls.kitasato-u.ac.jp) using primers by PCR method SEQ ID NO.2: 5'-GCCCATATGACAGAGCCCGGTACGTCCGTG-3' and reverse primer SEQ ID NO.3: 5'
[0023] - GCACTAGTTCAGCTTGCCGACCGCGGGAC-3' amplified. The camA gene and camB gene in the vector pT7NS-camAB (International Patent No. US 2006 / 0234337A1, whose gene sequence is shown in SEQ ID NO.4) respectively encode the pseudomonas redoxin reductase and pseudomonas of Pseudomonas putida cytoredoxin. The PCR amplification product and the vector pT7NS-camAB were digested with Nde I and Spe I, and ligated to obtain the recombinant vector pET11::sav7469::camA-camB (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com