Intravascular stent and preparation method thereof
A technology of vascular stents and tubes, which is applied in the medical field, can solve problems such as lack of technical parameters, achieve good degradation time, and improve the effect of radial support force
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
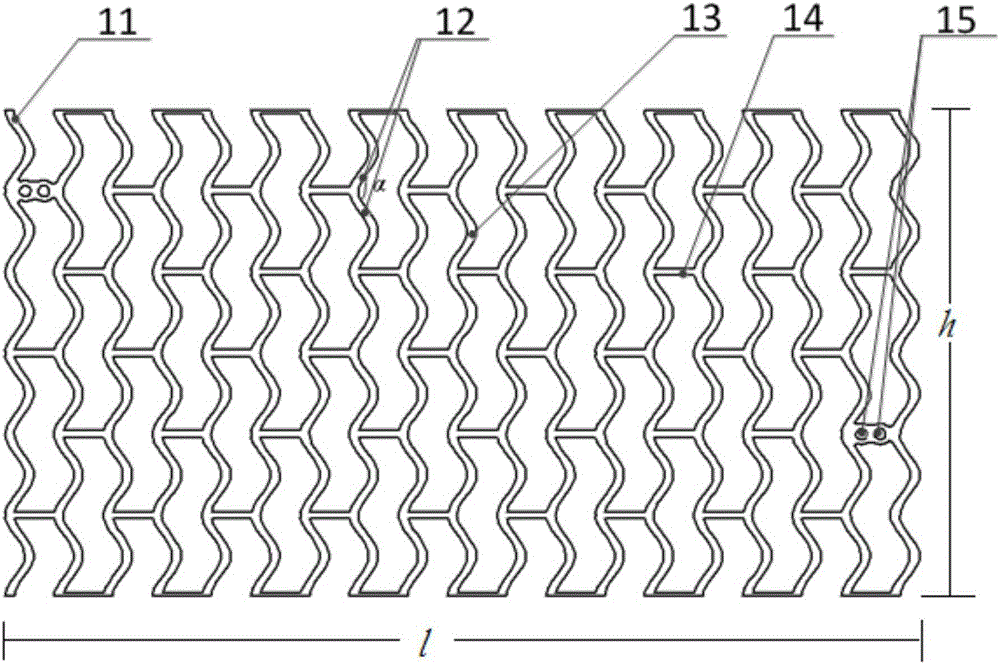
[0098] In this implementation case, 100% PLLA is selected as the raw material, and the PLLA particles are first put into a dryer connected online with the extruder, and dried at a temperature of 55°C for 5 hours to ensure that the water content of the PLLA particles is less than 150ppm. The extrusion process is carried out, the high temperature zone does not exceed 260 ° C, and the exit of the extrusion head is connected to a high vacuum sizing sleeve. Extrusion gives raw tubing.
[0099] Put the original pipe into the mold, seal one end of the pipe, fill the other end with 100PSI dry nitrogen and maintain a constant pressure, and heat the pipe to 100°C, and then the pipe will begin to expand radially after reaching the temperature, and at the same time Expansion (stretching) in the axial direction According to the appropriate axial stretching ratio of 150%, the pipe material is a pipe material with an outer diameter of 3.4mm and an inner diameter of 3.16mm, and the defined ra...
Embodiment example 2
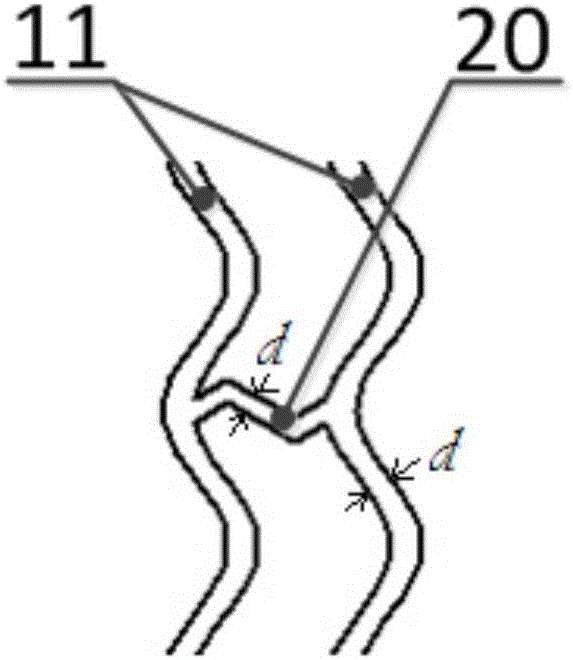
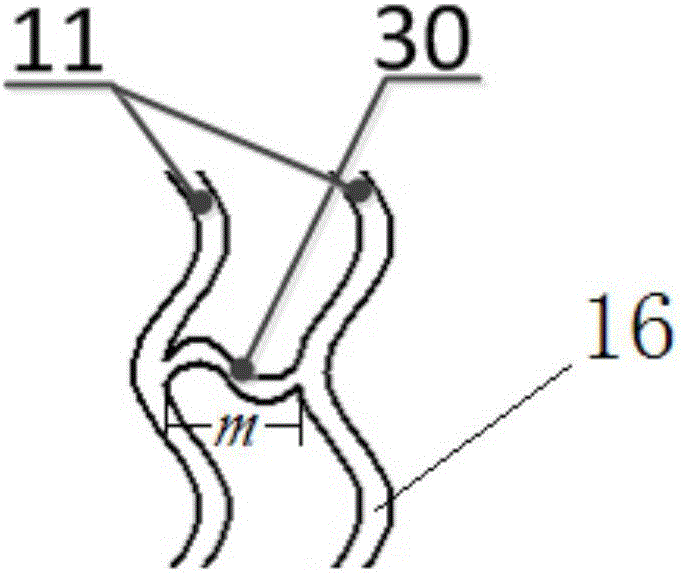
[0102] In this implementation case, 80% PLLA and 20% PLGA are selected as raw materials. According to the steps of Case 1, the radial expansion ratio is 200%, and the axial expansion (stretch) ratio is 200%. The outer diameter is 4.0mm and the inner diameter is . 3.54mm expansion tubing. At this time, laser processing was used to process the stent to have 16 ring structures with a width of 240 μm; each ring structure had 16 peaks; the number of connecting rods connecting two adjacent ring structures was 4, and the width was 160 μm. The length is 1.4mm; the ratio of the outer surface area of the stent to the plane area formed after the stent is expanded is 31%; micropores are arranged on the outer surface of the stent, the diameter of the micropore is 30 μm, and the depth is 1 / 2 of the wall thickness of the stent; The ratio of the sum of the areas on the outer surfaces to the area of the outer surface of the stent was 5%. According to the pipe forming process, the degradat...
Embodiment example 3
[0104] In this implementation case, 90% PLLA and 10% PLGA are selected as raw materials, and the outer diameter is 2.5mm, and the inner diameter is 2.5mm under the parameters of radial expansion ratio of 400% and axial expansion ratio of 10%. The 2.2mm expanded tubing is then processed by laser to obtain a scaffold structure, which has 12 ring structures with a width of 180 μm; each ring structure has 10 crests; the number of connecting rods connecting two adjacent ring structures is 3 pieces, with a width of 120 μm and a length of 1 mm; the ratio of the outer surface area of the stent to the plane area formed after the stent is deployed is 28%. At this time, micropores are set on the outer surface of the stent with a diameter of 20 μm and a depth equal to the wall thickness of the stent 1 / 3, the ratio of the total area of the micropores on the outer surface of the stent to the area of the outer surface of the stent is 3%. After testing, its radial support force can reac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com