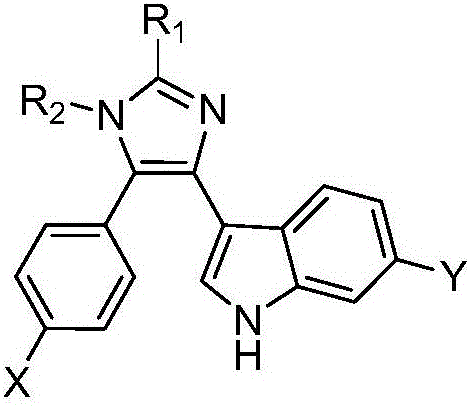
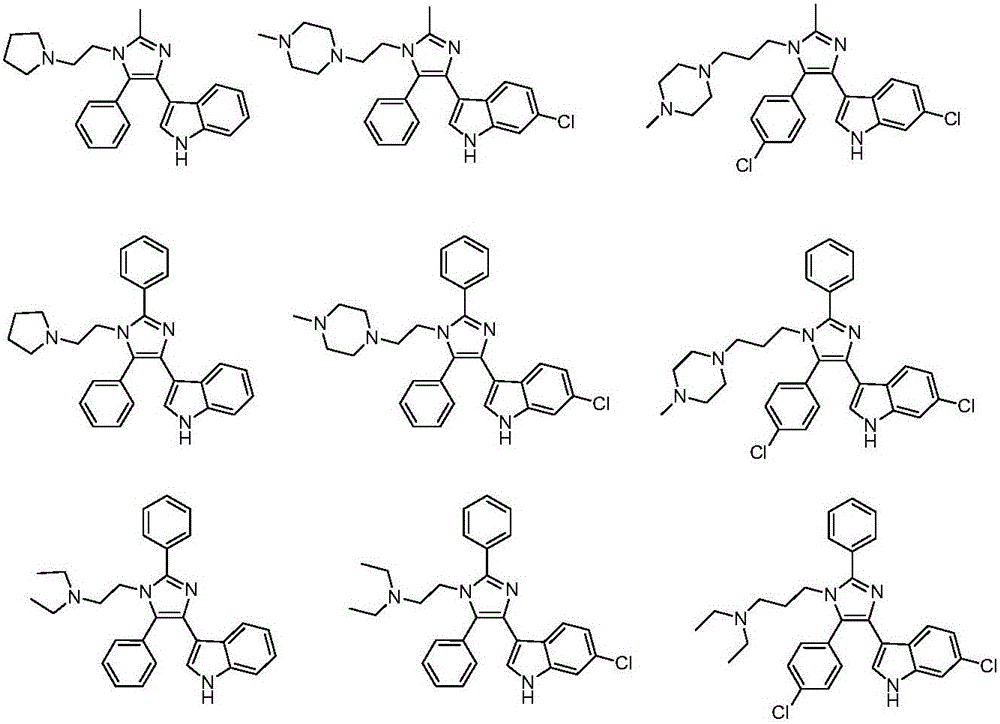
3-(4-phenyl-1H-imidazolyl-5-yl)-1H-indole derivatives, and preparation method and application thereof
A technology of indole derivatives and phenyl, which is applied in the field of 3--1H-indole derivatives and its preparation, can solve the problems of insufficient curative effect, large toxic and side effects, and low cure rate, and achieve good proliferation activity, Good anti-tumor cell proliferation activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
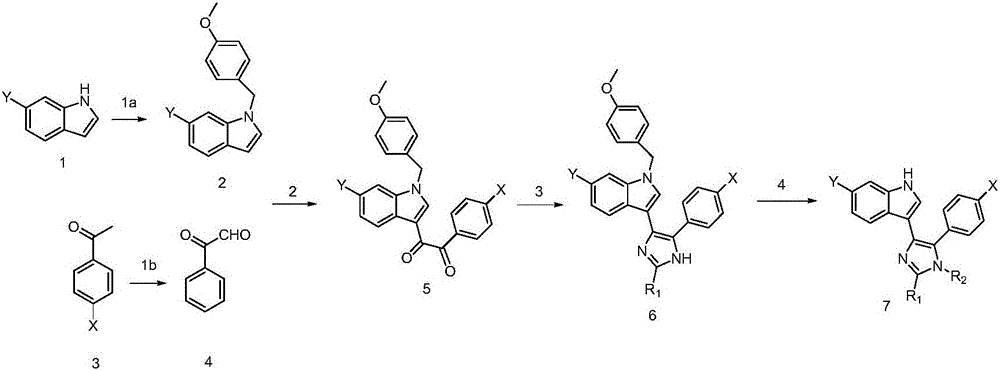
Examples
Embodiment 1
[0042] Example 1: Preparation of 6-chloro-1-(4-methoxy)-1H-indole.
[0043]
[0044]Sodium ethoxide solid (6.5g, 64mmol) was dissolved in DMF (25mL), stirred in an ice bath at 4°C, 6-chloro-indole (6.5g, 43mmol) was slowly dissolved in DMF (10mL), dripped at normal pressure A solution of p-methoxybenzyl chloride (6.5 mL, 47 mmol) in DMF (5.0 mL, 65 mmol) was added to the funnel. The reaction was carried out at room temperature, and the reaction was monitored by TLC until the raw materials were completely reacted. Pour into water, extract with ethyl acetate, take the ethyl acetate layer, wash with saturated brine, dry over anhydrous sodium sulfate, recover the solvent, and perform silica gel column chromatography on the obtained crude product with ethyl acetate:petroleum ether ratio of 1:12, 7.0 g of the product was obtained with a yield of 60.05%. m / z=271.08[M+H] + .
Embodiment 2
[0045] Embodiment 2: Preparation of chlorine-substituted 2-oxo-2-phenylacetaldehyde
[0046]
[0047] Dimethylsulfoxide (80.03mL, 1126mmol) and 4.25mL 1-(4-chlorophenyl)ethanone were poured into a round bottom flask and shaken gently. Iodine solid (16.5 g, 66 mmol) was quickly added, shaken gently, and dissolved. Add the remaining dimethyl sulfoxide, stir in an 80°C oil bath, and reflux for about 1 hour. The reaction was monitored by TLC until the starting material was completely reacted. Pour into water, dissolve 31.4g of sodium thiosulfate pentahydrate solid in 168.6g of water, stir to dissolve, and pour into the mixed solution. Ethyl acetate was separated and extracted, and the ethyl acetate layer was taken, washed with saturated brine, and dried over anhydrous sodium sulfate. The solvent was recovered, and the crude product was recrystallized in a solvent of ethyl acetate:petroleum ether at a ratio of 1:12. Vacuum filtration under reduced pressure yielded the pure p...
Embodiment 3
[0048] Example 3: Preparation of 1‐(6‐chloro‐1‐(4‐methoxybenzyl)‐1H‐indol‐3‐yl)‐2‐(4‐chlorophenyl)ethane‐1,2‐ diketone.
[0049]
[0050] Compound 2 (2.0 g, 7 mmol) and compound 4 (1.0 g, 6 mmol) were dissolved in toluene (31.3 mL) and stirred in an oil bath at 110°C. The reaction was monitored by TLC until the starting material was completely reacted. Spin dry to recover toluene to obtain a black viscous reaction product, add water, extract with ethyl acetate, collect the ethyl acetate layer, wash with saturated brine, dry over anhydrous sodium sulfate, and recover the solvent to obtain a crude product. The polarity of the solvent (ethyl acetate:petroleum ether: 1:15) was increased to 1:5, and silica gel column chromatography was performed to obtain 1.0 g of the product with a yield of 40.74%.
[0051] Product Confirmation: m / z=437.06[M+H] + ; 1 H NMR (400MHz, CDCl 3 ): δ: 7.238(dd, J=2.69Hz, 2H, Ar-H), 7.020-7.080(m, J=11.89Hz, 3H, Ar-H), 6.808-6.845(d, J=4.03Hz, 4H ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com