Application of 183aa micro-molecule polypeptides
A small molecule polypeptide, 1.183aa technology, applied in the application field of 183aa small molecule polypeptide, to achieve the effect of promoting cell apoptosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1183aa
[0016] Example 1 183aa polypeptide recombination
[0017] 1) Cloning vector construction of recombinant peptide: extract human mRNA, reverse transcribe it into cDNA, use cDNA as template, upstream primer is 5'-GGGGTACCATGCACCTGCTCCCTCTCTGG-3' (containing KPN I restriction site); downstream primer is 5'- CGGGATCCTACTGTGTTCATCATACTGTCGAA-3' (with Bamh I restriction site).
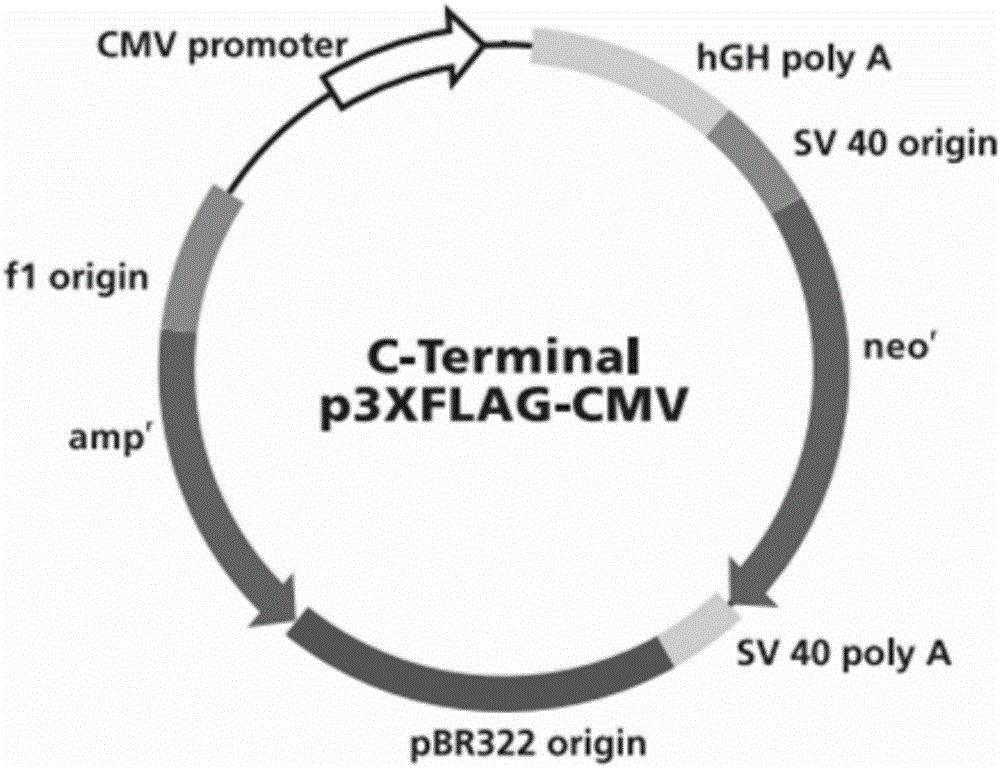
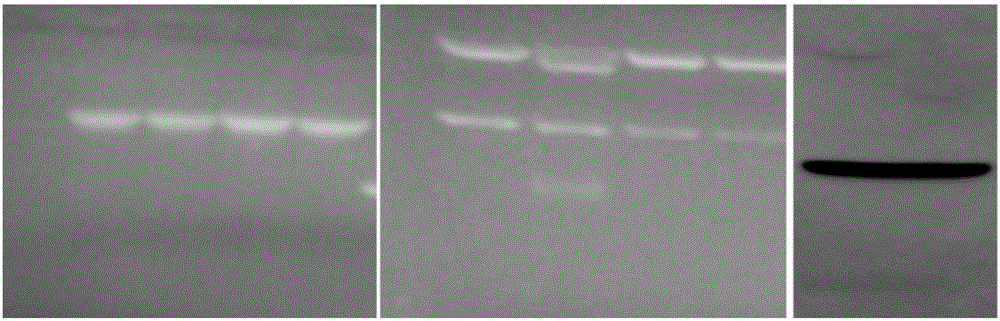
[0018] The 552bp mRNA sequence corresponding to 183aa was successfully amplified by PCR technology, as shown in SEQ ID NO.2, and the amino acid sequence of the expressed 183aa small molecule polypeptide was shown in SEQ ID NO.1. The amplified fragment was ligated with the p3×FLAG-CMV-14 vector ( figure 1 ), transfer the ligated product into competent Escherichia coli DH5α, select clones on the Amp-containing agar plate, extract the recombinant plasmid with alkaline lysis method, and identify it with KPN I and Bamh I enzymes.
[0019] The main process of the above operation is as follows:
[0020] The STAT1...
Embodiment 2
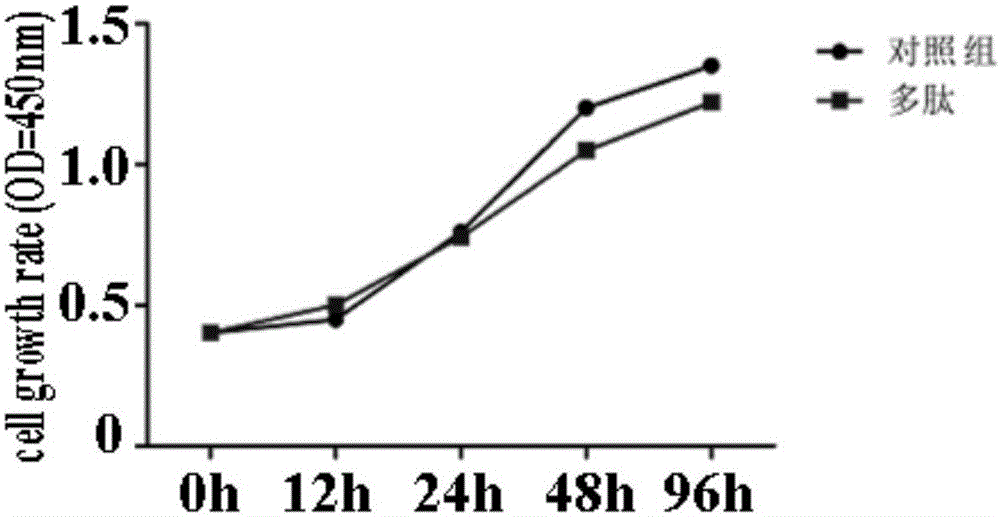
[0028] Example 2 Detection of Anti-Multiple Myeloma Function of Recombinant Peptides
[0029] 1) CCK-8 experiment proves the anti-proliferation effect of recombinant peptides on multiple myeloma cells at the cellular level
[0030] CCK-8 cell proliferation experiment: In RPMI 8226 cells, the cells were collected 48 hours after transfection with wild-type and 183aa fragment truncated STAT1 vectors, and 5000 cells per well were seeded into 96-well plates with a volume of 100 μL per well. Change the medium in each well (the ratio of CCK-8 to culture medium is 1:10), incubate at 37°C for 2 hours, change the medium after reaching the time point, add 100 μL of cell culture medium containing 10 μL of CCK-8 solution to each well (be careful not to Air bubbles are formed in the well and they will affect the O.D reading). Incubate the culture plate in the incubator for 1.5-2h. (The same time should be kept at different time points) Measure the absorbance at (measurement wavelength 490...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com