Method for synthesizing benzoyl peroxide and benzoyl peroxide synthesized with method
A technology of benzoyl peroxide and benzoyl chloride, applied in the field of benzoyl peroxide and synthesizing benzoyl peroxide, can solve the problems of uneven product particle size, product inclusion of impurities, excessive chloride ions, etc. The effect of uniform particle size, low production cost and easy washing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
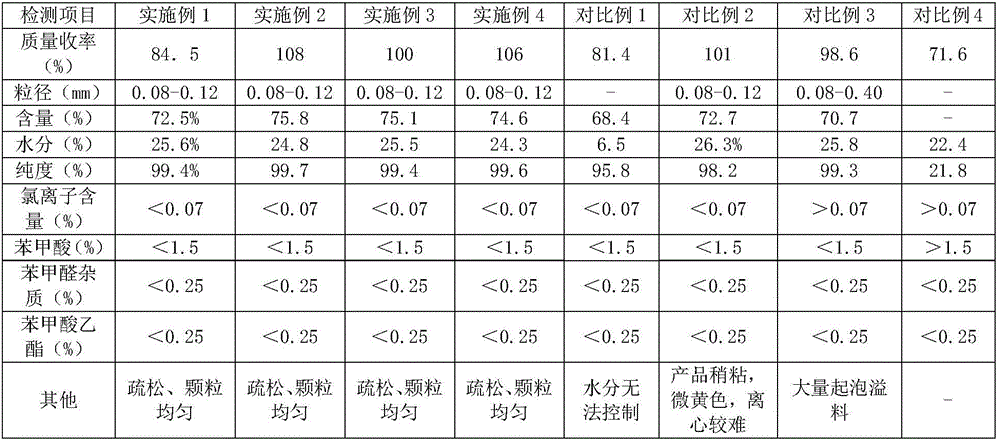
Embodiment 1
[0042] The method for the synthesis of benzoyl peroxide provided by this embodiment comprises the following steps:
[0043] S11. At room temperature, dissolve the surfactant in the reaction solvent and stir, then add 15.65kg of sodium hydroxide and stir until dissolved to obtain a solution. Keep the temperature of the solution between -5 and 5°C, and add 6.04kg of it dropwise Hydrogen peroxide, insulation and stirring after completion of dripping, to obtain sodium peroxide solution;
[0044] S12. Keep the temperature of the sodium peroxide solution between -5 and 5°C, then add 50kg of benzoyl chloride dropwise, keep warm and stir after the dropwise addition, centrifuge the reacted solution, and wash the centrifuged solid with water , to obtain benzoyl peroxide.
Embodiment 2
[0046] The method for the synthesis of benzoyl peroxide provided by the present embodiment specifically comprises the following steps:
[0047]S21. At room temperature, dissolve 150g of sodium lauryl sulfate in 120kg of water, stir for 30 minutes, then add 18.5kg of sodium hydroxide and stir until dissolved to obtain a solution. Keep the temperature of the solution at -5 to 5°C. And dropwise add 24kg mass fraction is 30% hydrogen peroxide, keep warm and stir for 1 hour after the drop is completed, after fully reacting, obtain sodium peroxide solution;
[0048] S22. Keep the temperature of the sodium peroxide solution between -5 and 5°C, then add 50kg of benzoyl chloride dropwise, keep warm and stir for 1 hour after the dropwise addition, centrifuge the reacted solution, and centrifuge the centrifuged solid Carry out washing, obtain benzoyl peroxide product 54kg.
Embodiment 3
[0050] The method for the synthesis of benzoyl peroxide provided by the present embodiment specifically comprises the following steps:
[0051] S31. At room temperature, dissolve 40 g of sodium lauryl sulfate in 24 kg of water, stir for 30 minutes, then add 4.2 kg of sodium hydroxide and stir until dissolved to obtain a solution, and keep the temperature of the solution at -5 to 5°C. And add dropwise 5.5kg mass fraction of 30% hydrogen peroxide, keep warm and stir for 0.5 hour after the drop is completed, after fully reacting, obtain sodium peroxide solution;
[0052] S32. Keep the temperature of the sodium peroxide solution between -5 and 5°C, then add 10 kg of benzoyl chloride dropwise, keep warm and stir for 0.5 hours after the dropwise addition, centrifuge the reacted solution, and centrifuge the centrifuged solid Carry out washing, obtain 10kg benzoyl peroxide product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com