Medicinal preparation for preventing and treating postpartum diseases of sows and preparation method thereof
A technology for postpartum diseases and pharmaceutical preparations, applied in sexual diseases, drug combinations, pharmaceutical formulations, etc., can solve the problems of yellow and white diarrhea of piglets, harm to the pig industry, disturbing the beneficial flora of sows, etc. Broad application prospects and the effect of regulating ecological balance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
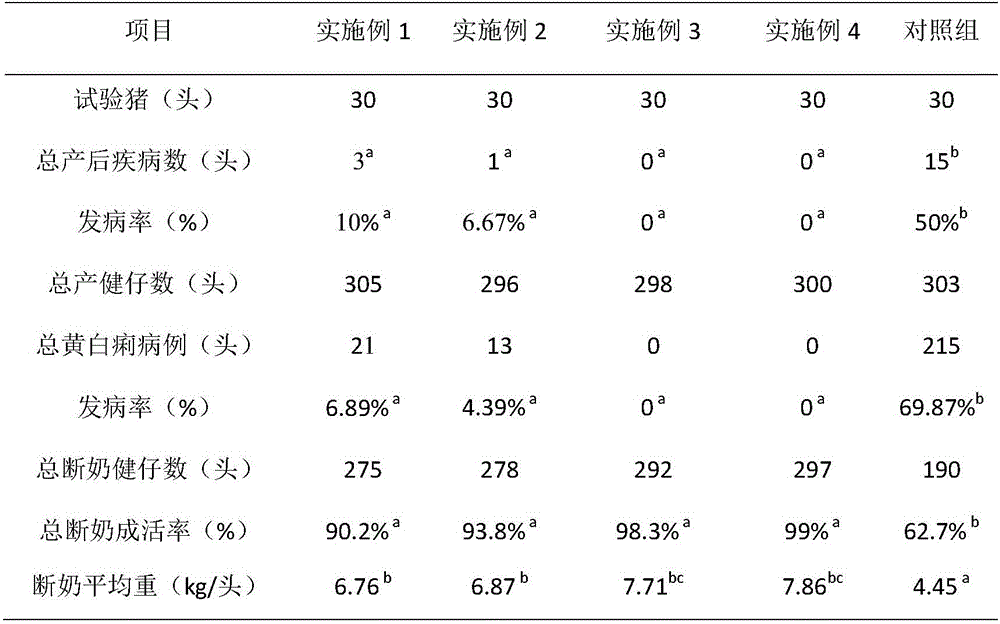
[0018] A pharmaceutical preparation for preventing and treating postpartum diseases of sows, characterized in that it is prepared from the following ingredients in parts by weight: 5 parts of lincomycin hydrochloride, 2 parts of ciprofloxacin lactate, 1 part of trimethoxine sodium lactate, and honeysuckle 8 parts, 5 parts of Houttuynia cordata, 1 part of Passepartout, 20 parts of medical stone, 2 parts of fructooligosaccharide, 0.5 part of citric acid.
[0019] A pharmaceutical preparation for preventing and treating postpartum diseases of sows, comprising the following steps:
[0020] 1. Weigh honeysuckle, Houttuynia cordata, Passepartout, medical stone, fructooligosaccharide, citric acid, ultrafinely pulverize, pass through a 150-mesh sieve, and make auxiliary materials.
[0021] 2. Weigh lincomycin hydrochloride, ciprofloxacin lactate, and trimethoxine sodium lactate, and mix well.
[0022] 3. Fully mix step 1 and step 2 evenly to obtain the pharmaceutical preparation of t...
Embodiment 2
[0024] A pharmaceutical preparation for preventing and treating postpartum diseases of sows, characterized in that it is prepared from the following ingredients in parts by weight: 7 parts of lincomycin hydrochloride, 3 parts of ciprofloxacin lactate, 2 parts of trimethoxine sodium lactate, honeysuckle 10 parts, 7 parts of Houttuynia cordata, 2 parts of Passepartout, 23 parts of medical stone, 3 parts of fructooligosaccharide, 1 part of citric acid.
[0025] A pharmaceutical preparation for preventing and treating postpartum diseases of sows, comprising the following steps:
[0026] 1. Weigh honeysuckle, Houttuynia cordata, Passepartout, medical stone, fructooligosaccharide, citric acid, ultrafinely pulverize, pass through a 150-mesh sieve, and make auxiliary materials.
[0027] 2. Weigh lincomycin hydrochloride, ciprofloxacin lactate, and trimethoxine sodium lactate, and mix well.
[0028] 3. Fully mix step 1 and step 2 evenly to obtain the pharmaceutical preparation of the ...
Embodiment 3
[0030] A pharmaceutical preparation for preventing and treating postpartum diseases of sows, characterized in that: it is prepared from the following ingredients in parts by weight: 8 parts of lincomycin hydrochloride, 5 parts of ciprofloxacin lactate, 5 parts of trimethoxine sodium lactate, honeysuckle 12 parts, 10 parts of Houttuynia cordata, 5 parts of Passepartout, 30 parts of medical stone, 5 parts of fructooligosaccharide, 2 parts of citric acid.
[0031] A pharmaceutical preparation for preventing and treating postpartum diseases of sows, comprising the following steps:
[0032] 1. Weigh honeysuckle, Houttuynia cordata, Passepartout, medical stone, fructooligosaccharide, citric acid, ultrafinely pulverize, pass through a 150-mesh sieve, and make auxiliary materials.
[0033] 2. Weigh lincomycin hydrochloride, ciprofloxacin lactate, and trimethoxine sodium lactate, and mix well.
[0034] 3. Fully mix step 1 and step 2 evenly to obtain the pharmaceutical preparation of t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com