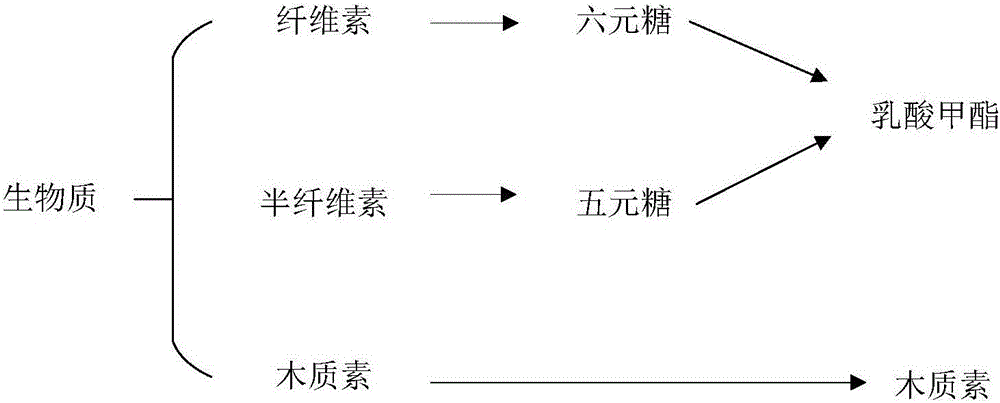
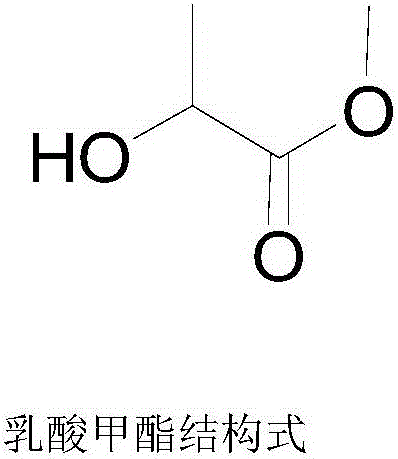
Method for preparing methyl lactate through supported nickel oxide catalyzed monosaccharide conversion in near critical methanol medium
A methyl lactate, supported technology, applied in the field of methyl lactate prepared from supported nickel oxide catalyzed conversion of monosaccharides in near-critical methanol medium, can solve the problems of difficult industrialization, difficult loading of nano-nickel oxide, long preparation cycle, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
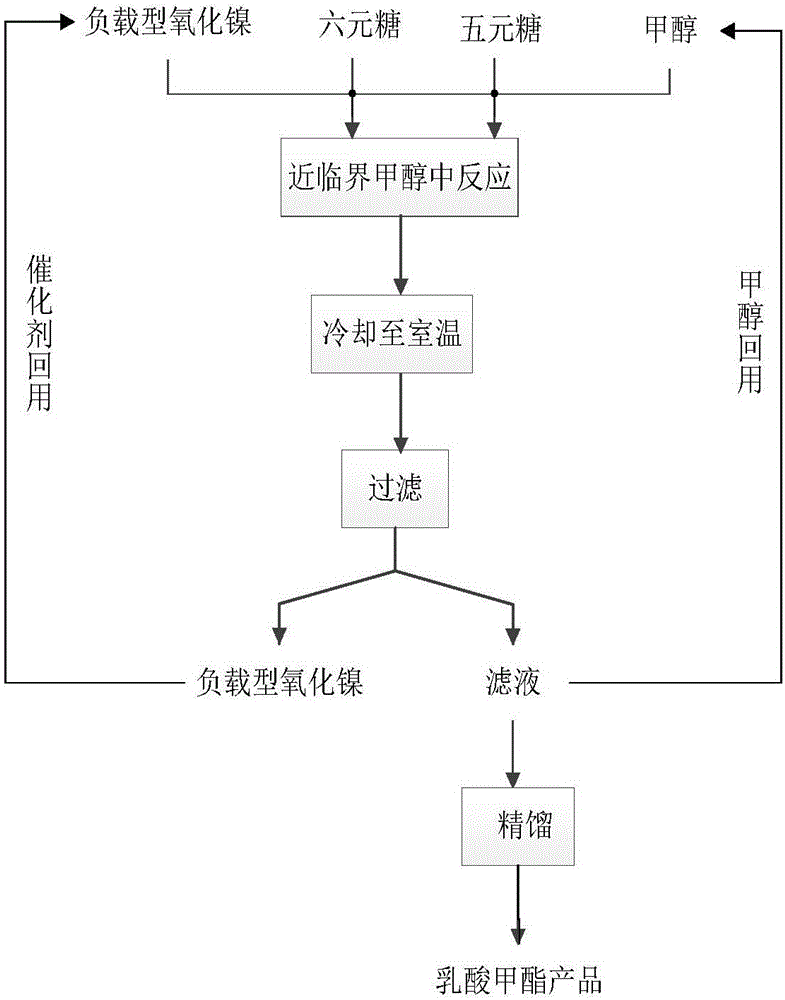
[0034] First add 1.5g glucose and 1.5g xylose to a 500mL stirred high temperature and high pressure reactor, then add 300mL methanol, the total mass concentration of glucose and xylose is 10g / L, and finally add 0.75gNiO / ZrO 2 , the total mass of glucose and xylose and NiO / ZrO 2 The mass ratio is 4:1; start stirring, heat up to 160°C, and the reaction time is 12h; after the reaction, cool down to room temperature, filter, and filtrate (the mass yield of methyl lactate is 38.27% by GC analysis after sampling) The methyl lactate product is obtained after rectification, and the methanol is reused; the filter residue is obtained as a supported nickel oxide catalyst, which is washed with methanol, dried, and reused.
Embodiment 2
[0036]First add 4.5g glucose and 4.5g xylose to a 500mL stirred high-temperature and high-pressure reactor, then add 300mL methanol, the total mass concentration of glucose and xylose is 30g / L, and finally add 2.25gNiO / ZrO 2 , the total mass of glucose and xylose and NiO / ZrO 2 The mass ratio is 4:1; start stirring, heat up to 160°C, and the reaction time is 12h; after the reaction, cool down to room temperature, filter, and filtrate (after sampling, the mass yield of methyl lactate is 38.66% by GC analysis) The methyl lactate product is obtained after rectification, and the methanol is reused; the filter residue is obtained as a supported nickel oxide catalyst, which is washed with methanol, dried, and reused.
Embodiment 3
[0038] First add 7.5g glucose and 7.5g xylose to a 500mL stirred high-temperature and high-pressure reactor, then add 300mL methanol, the total mass concentration of glucose and xylose is 50g / L, and finally add 3.75gNiO / ZrO 2 , the total mass of glucose and xylose and NiO / ZrO 2 The mass ratio is 4:1; start stirring, heat up to 160°C, and the reaction time is 12h; after the reaction is completed, cool down to room temperature, filter, and filtrate (the mass yield of methyl lactate is 37.05% by GC analysis after sampling) The methyl lactate product is obtained after rectification, and the methanol is reused; the filter residue is obtained as a supported nickel oxide catalyst, which is washed with methanol, dried, and reused.
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com