4-hydroxyl pyrroline-2-ketone derivative containing 1,3,4-oxadiazole and preparation method and application of 4-hydroxyl pyrroline-2-ketone derivative
A technology of hydroxypyrroline and oxadiazole, which is applied in the field of chemistry and technology, can solve problems such as compound instability, and achieve good inhibitory activity and stable compound effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
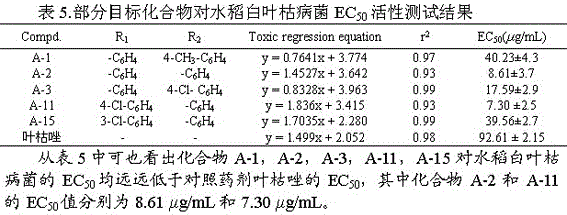
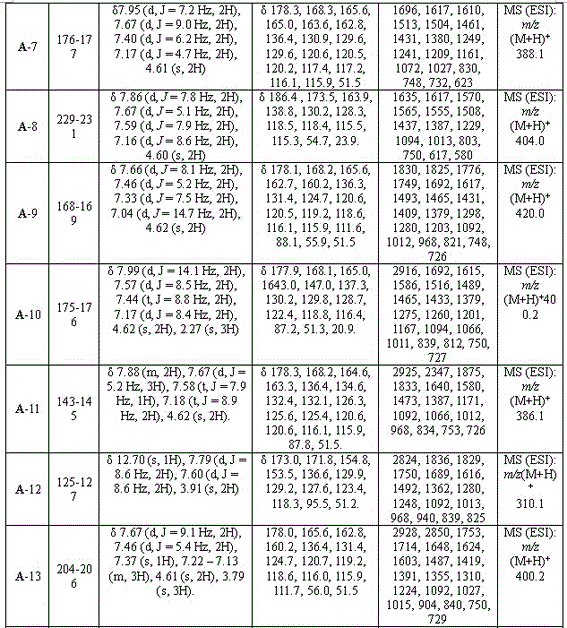
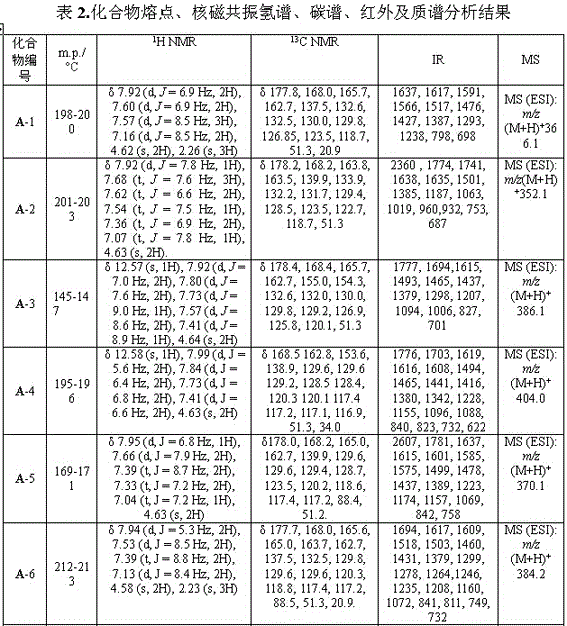
[0027] Example 1: 3-(5-(phenyl)-1,3,4-oxadiazol-2-sulfanyl)-1-(4-methylphenyl)-4-hydroxy-pyrroline-2-one (A-1), including the following steps:
[0028] (1) Preparation of ethyl benzoate
[0029] Add 2.0 g (16.38 mmol) of benzoic acid to a 100 mL round bottom flask, add 50 mL of ethanol to dissolve, add 1 mL (20.1 mmol) of concentrated sulfuric acid dropwise, heat and reflux for 2 h, TLC detects that the reaction is complete, and adjust the pH with saturated sodium carbonate solution to about 7, extracted with dichloromethane (30mL×3), combined the organic phases, dried over anhydrous sodium sulfate, precipitated, and column chromatography (PE:EA=10:1, V / V ) to obtain 2.2 g of colorless oily liquid, yield 89.4%;
[0030] (2) Preparation of Benzohydrazide
[0031] Add 2 g (13.32 mmol) of ethyl benzoate to a 100 mL round bottom flask, 50 mL of absolute ethanol as a solvent, add 1.00 g (19.98 mmol) of 80% hydrazine hydrate, and place the system in an oil bath at a temperature ...
Embodiment 2
[0043] Example 2: 3-(5-(phenyl)-1,3,4-oxadiazol-2-sulfanyl)-1-phenyl-4-hydroxy-pyrroline-2-one (A-2)
[0044] (1) Preparation of ethyl benzoate
[0045] As embodiment 1 (1) method and condition synthesis,
[0046] (2) Preparation of Benzohydrazide
[0047] Synthesize as embodiment 1 (2) method and condition.
[0048] (3) Preparation of 5-(phenyl)-1,3,4-oxadiazole-2-thiol
[0049] Synthesized as in Example 1 (3) method and conditions.
[0050] (4) N Preparation of -(4-phenyl)-glycine ethyl ester
[0051] Synthesized as in Example 1 (4) method and conditions, replacing (18.66 mmol) 4-methylaniline with (21.48 mmol) aniline.
[0052] (5) N -(2-Bromoacetyl)- N -(Phenyl)glycine ethyl ester
[0053] Synthesize as embodiment 1 (5) method and condition, (15.52 mmol) N -(p-tolyl)glycine ethyl ester was changed to (16.76 mmol) N -(p-Clyl)glycine ethyl ester
[0054] (6) N -(2-Bromoacetyl)- N Preparation of -phenyl-(2-(5-phenyl-1,3,4-oxadiazole-2-mercapto)acetyl)glycine eth...
Embodiment 3
[0058] Example 3: 3-(5-(4-fluorophenyl)-1,3,4-oxadiazole-2-thio)-1-(4-chlorophenyl)-4-hydroxy-pyrroline- 2 Ketones (A-4)
[0059] (1) Preparation of ethyl 4-fluorobenzoate
[0060] Synthesize as in Example 1 (1) method and conditions, the difference is that (16.38 mmol) benzoic acid is replaced by (14.27 mmol) 4-fluorobenzoic acid
[0061] (2) Preparation of 4-fluorobenzoic hydrazide
[0062] Synthesize as in Example 1 (2) method and conditions, the difference is that (13.32 mmol) ethyl benzoate is replaced with (11.89 mmol) 4-fluoroethyl benzoate.
[0063] (3) Preparation of 5-(4-fluorophenyl)-1,3,4-oxadiazole-2-thiol
[0064] Synthesize as in Example 1 (3) method and conditions, the difference is that (11.02 mmol) benzoyl hydrazide is replaced by (9.73 mmol) p-fluorobenzoic hydrazide.
[0065] (4) N Preparation of -(4-chlorophenyl)-glycine ethyl ester
[0066] Synthesize as in Example 1 (4) method and conditions, the difference is that adding (18.66 mmol) 4-methylanili...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com