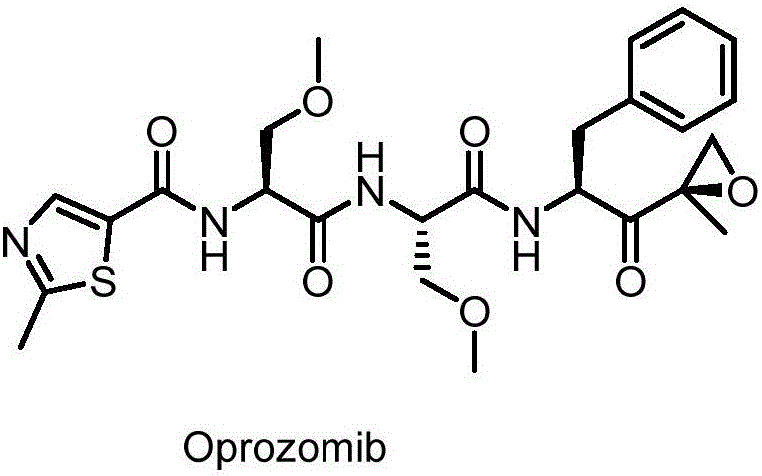
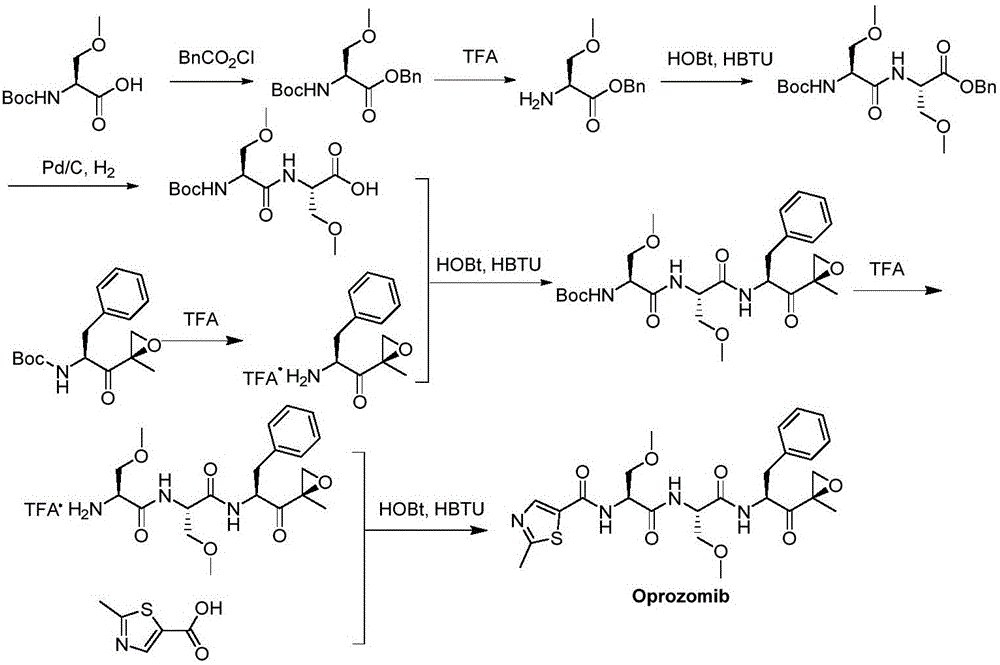
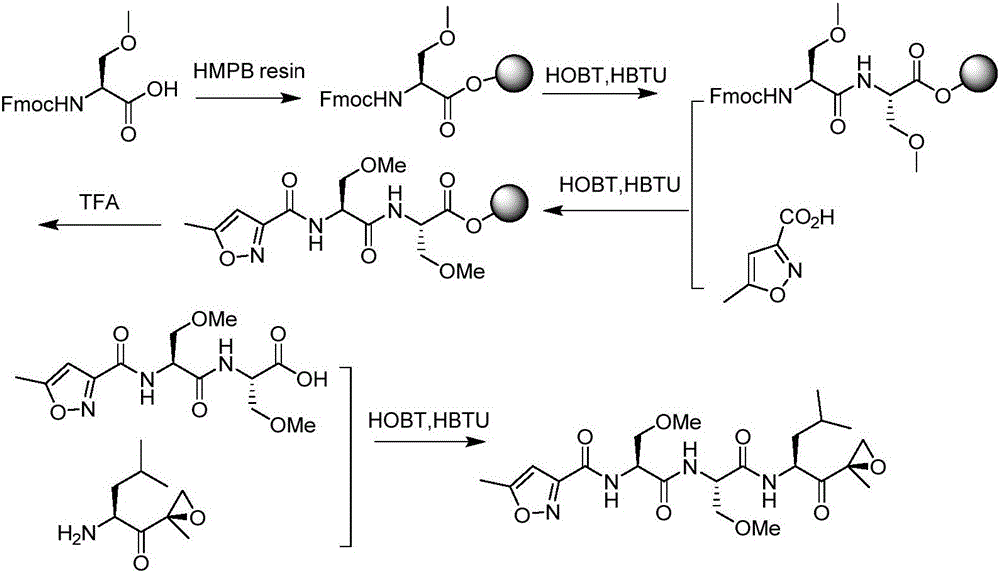
Method for preparing proteasome inhibitor Oprozomib and analogs thereof
A proteasome inhibitor and analog technology, which is applied in the field of preparation of proteasome inhibitor oprozomib and its analogs, can solve the problems of complicated operation, prolonged production cycle, loss of synthesized peptide chains, etc., and achieves improved yield and purity , the effect of shortening the reaction cycle and reducing the production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1 N-Fmoc-O-methyl-L-serine tert-butyl alcohol ester
[0066]
[0067] Dissolve N-Fmoc-O-methyl-L-serine (1g, 2.93mmol) in 15mL of ethyl acetate, slowly add tert-butyl trichloroacetimide ester (1.28 g, 5.87mmol), the mixture was stirred at 25°C for 18 hours, the reaction was quenched with saturated sodium carbonate (20mL), the organic layer was separated, and the organic layer was washed twice with saturated brine, dried over anhydrous sodium sulfate, filtered, and reduced The solvent was evaporated under pressure, and 1.13 g of white solid was obtained by column chromatography.
[0068] Yield: 97%; 1 H NMR (500MHz, CDCl 3 ): δ7.76(d, J=7.5Hz, 2H), 7.62(q, J=4.0Hz, 2H), 7.40(t, J=7.5Hz, 2H), 7.31(t, J=7.4Hz, 2H ), 5.66(d, J=8.0Hz, 1H), 4.417-4.331(m, 3H), 4.24(t, J=7.5Hz, 1H), 3.78(dd, J=9.5, 3.5Hz, 1H), 3.65 (dd, J=9.0, 3.0Hz, 1H), 3.37(s, 3H), 1.48(s, 9H).ESI-MS: m / z=398[M+1] + .
Embodiment 2
[0069] Example 2 O-methyl-L-serine tert-butanol ester
[0070]
[0071] The N-Fmoc-O-methyl-L-serine tert-butanol ester (1g, 2.52mmol) obtained in Example 1 was dissolved in 9mL of acetonitrile, and 1mL of diethylamine was added under an ice bath, and detected by thin layer chromatography, the reaction After completion, the solvent was recovered under reduced pressure, and the white solid crude product was 440 mg, which was directly used in the next step.
[0072] Yield: 100%; 1 H NMR (500MHz, CDCl 3 ): δ4.24(t, J=7.5,1H),3.81(dd,J=9.5,3.5Hz,1H),3.56(s,1H),3.67(dd,J=9.0,3.0Hz,1H), 3.37(s,3H), 3.12(s,1H), 1.47(s,9H).
Embodiment 3
[0073] Example 3 O-methyl-N-(2-methyl-5-thiazole)-L-serine tert-butyl ester
[0074]
[0075] Under ice-cooling, the amine obtained in Example 2 (440 mg, 2.5 mmol) and 2-methylthiazole-5-carboxylic acid (429 mg, 3 mmol) were dissolved in tetrahydrofuran (10 mL), and HOBt (135 mg, 3 mmol) and HBTU (1.1 g, 3mmol) was slowly added dropwise N,N-diisopropylethylamine ((1mL, 6mmol), raised to room temperature and reacted for 4 hours. Added 20mL of ethyl acetate and allowed to stand to separate the organic layer. Wash with ammonium solution, saturated sodium bicarbonate solution and saturated saline solution, dry over anhydrous sodium sulfate, filter, recover the solvent under reduced pressure to obtain a residue, and purify by silica gel column chromatography to obtain 0.6 g of white solid.
[0076] Yield: 80%; 1 H NMR (500MHz, CDCl 3 )δ8.04(s,1H),6.74(d,J=7.5Hz,1H),4.74-4.72(m,1H),3.81(dd,J=9.5,3.0Hz,1H),3.71(dd,J =9.5,3.0Hz,1H),3.35(s,3H),2.72(s,3H),1.48(s,9H).ESI-MS: m / z=30...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com