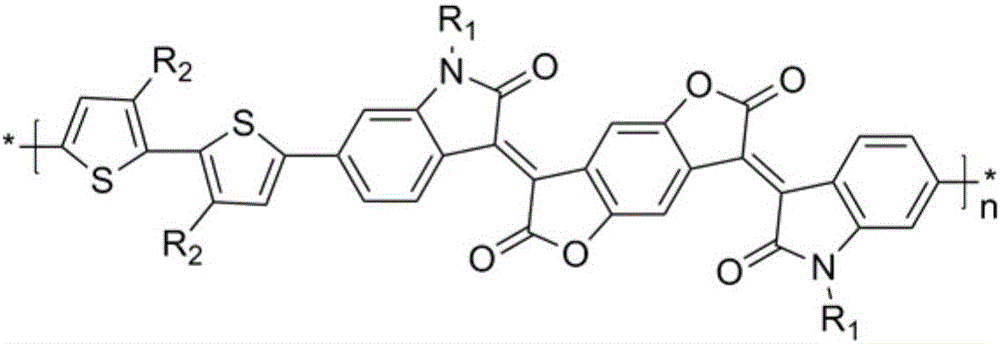
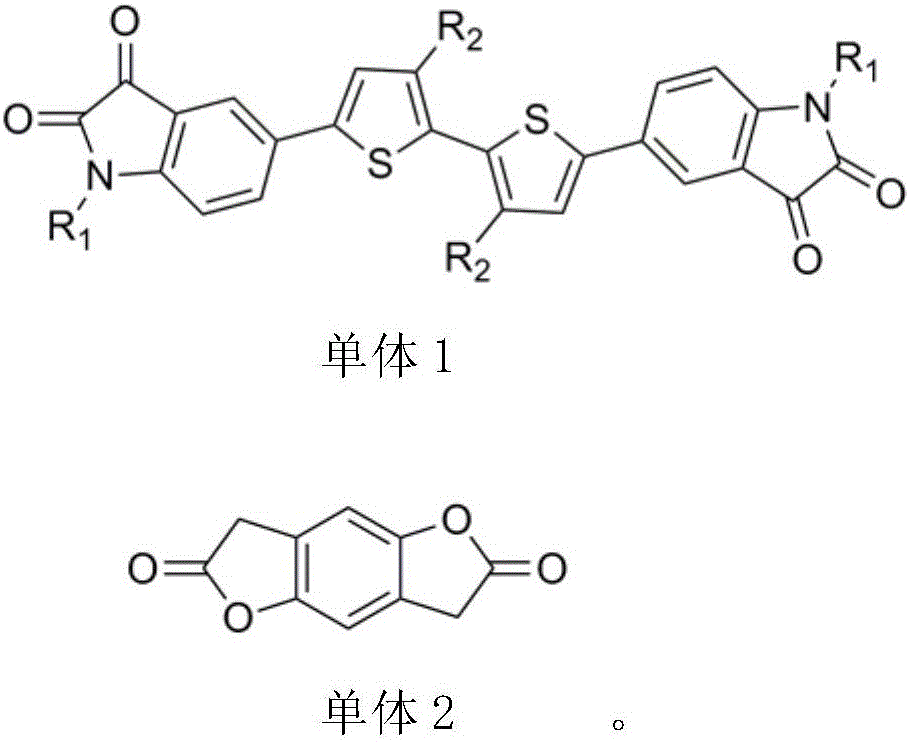
Novel polymerization method of conjugated polymer based on (2-oxyindole-3-idene) benzodifuran-dione and bithiophene
A benzodifuran and conjugated polymer technology, which is applied in the field of organic polymer preparation, can solve the problems of high reaction conditions, toxic and expensive catalysts, etc., and achieves low reaction cost, high atom utilization, and efficient preparation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0018] The preparation method of each monomer is described as follows:
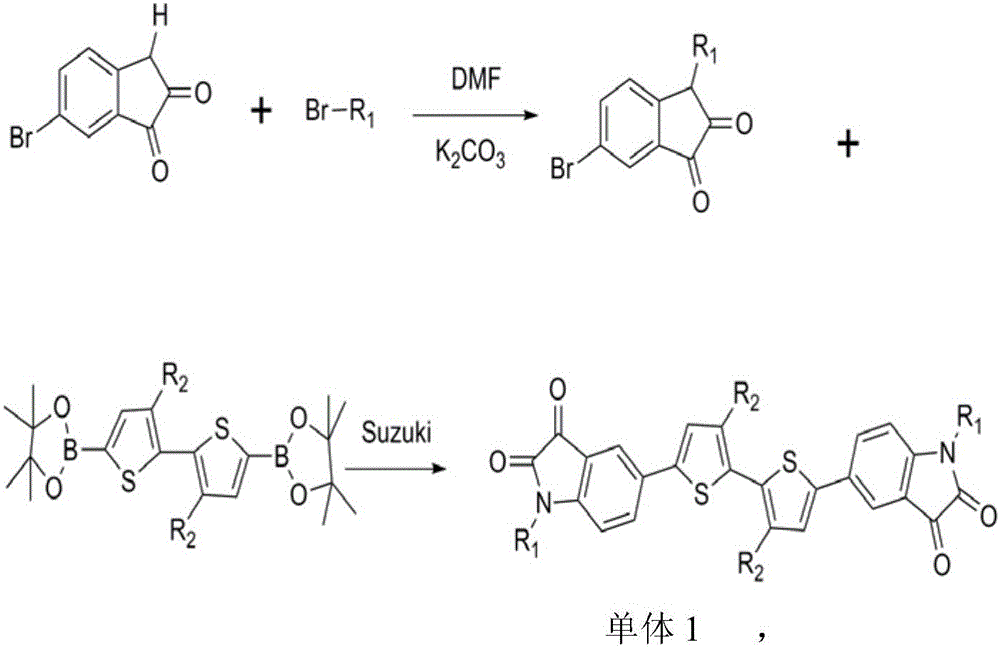
[0019] The specific steps for the synthesis of monomer 1 thiophene dibenzopyrrole diketone: hexabromoisatin and thiophene boron monomers can be purchased directly, and hexabromoisatin and potassium carbonate react with brominated alkyl chains in DMF The obtained compound is then reacted with thiophene boron monomer to obtain monomer 1 through conventional Suzuki cross-coupling reaction. The synthetic route is shown as follows:
[0020]
[0021] Synthetic steps for preparing monomer 2 benzodifurandione: benzodifurandione monomer is prepared by literature method, detailed preparation method can be found in literature report (Journal of the American Chemical Society, 135(33), 12168-12171 ; 2013), the synthetic route of monomer benzodifurandione is shown as follows:
[0022]
Embodiment 1
[0023] Embodiment 1, synthetic polymer P1
[0024] The specific steps for the synthesis of polymer P1 are as follows: add 0.180g (0.123mmol) of monomer 1 and 0.023g (0.123mmol) of monomer 2 into a 100mL polymerization reaction tube, and then add 0.06g (0.315mmol) of toluenesulfonic acid as a catalyst, After dissolving the above substances with 5mL of toluene, use N 2 The air was exhausted for 15 minutes, and the reaction was sealed at 120°C for 48 hours. Cool the reaction to room temperature, add 200mL of methanol to precipitate, filter the solid, extract with methanol and n-hexane Soxhlet for 24 hours, and then use chloroform for 24 hours, and finally rotate the liquid, and precipitate with methanol to obtain a polymer, polymer P1 The synthesis path is shown as follows:
[0025]
Embodiment 2-3
[0027] The specific steps are the same as in Example 1: add 0.123mmol monomer 1 and 0.123mmol monomer 2 in the 100mL polymerization reaction tube, then add catalyst 0.315mmol toluenesulfonic acid (catalyst amount can be changed correspondingly according to solvent amount and reaction time), use After dissolving the above substances in 5mL toluene, use N 2 The air was exhausted for 15 minutes, and the reaction was sealed at 120°C for 48 hours. Cool the reaction to room temperature, add 200mL of methanol to precipitate, filter the solid, extract with methanol and n-hexane Soxhlet for 24 hours, and then extract with chloroform for 24 hours, finally spin the liquid, and precipitate with methanol to obtain polymers P2-P3. The specific structure is shown in Table 1.
[0028] Table 1
[0029]
[0030] In summary, the present invention relates to a novel polymerization method based on a conjugated polymer of (2-oxindol-3-ylidene)benzodifuran-dione and thiophene. The D-A type con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com