Preparation method of hydroxylapatite fluorescent material doped with rare earth samarium
A technology of hydroxyapatite and fluorescent materials, applied in the direction of luminescent materials, chemical instruments and methods, etc., can solve problems such as hydroxyl quenching, and achieve the effects of low cost, high luminous intensity, and cheap and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
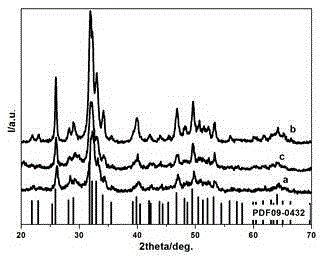
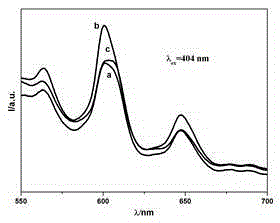
Embodiment 1 150
[0025] Example 1 (150°C hydrothermal reaction sample)
[0026] Weigh the calculated amounts of calcium nitrate 1.92 g, potassium dihydrogen phosphate 0.68 g, urea 2.10 g, and samarium nitrate 0.10 g, respectively, and add appropriate amount of distilled water to prepare calcium nitrate aqueous solution, potassium dihydrogen phosphate aqueous solution, urea aqueous solution and samarium nitrate aqueous solution . Under the condition of room temperature and stirring, the prepared above-mentioned solutions were mixed according to the order of calcium nitrate aqueous solution, potassium dihydrogen phosphate aqueous solution, samarium nitrate aqueous solution and urea aqueous solution.
[0027] Under stirring conditions at room temperature, ammonia water (5 mol / L) was added dropwise into the mixed solution, and the pH value of the solution was adjusted to about 10. Continue to stir the suspension for 0.5 h, and control the pH to no longer change to obtain a hydrothermal precursor....
Embodiment 2 120
[0029] Example 2 (120°C hydrothermal reaction sample)
[0030] Weigh the calculated amounts of calcium nitrate 1.92 g, potassium dihydrogen phosphate 0.68 g, urea 2.10 g, and samarium nitrate 0.10 g, respectively, and add appropriate amount of distilled water to prepare calcium nitrate aqueous solution, potassium dihydrogen phosphate aqueous solution, urea aqueous solution and samarium nitrate aqueous solution . Under the condition of room temperature and stirring, the prepared above-mentioned solutions were mixed according to the order of calcium nitrate aqueous solution, potassium dihydrogen phosphate aqueous solution, samarium nitrate aqueous solution and urea aqueous solution.
[0031] Under stirring conditions at room temperature, ammonia water (5 mol / L) was added dropwise into the mixed solution, and the pH value of the solution was adjusted to about 10. Continue to stir the suspension for 0.5 h, and control the pH to no longer change to obtain a hydrothermal precursor....
Embodiment 3 90
[0033] Example 3 (90°C hydrothermal reaction sample)
[0034] Weigh the calculated amounts of calcium nitrate 1.92 g, potassium dihydrogen phosphate 0.68 g, urea 2.10 g, and samarium nitrate 0.10 g, respectively, and add appropriate amount of distilled water to prepare calcium nitrate aqueous solution, potassium dihydrogen phosphate aqueous solution, urea aqueous solution and samarium nitrate aqueous solution . Under the condition of room temperature and stirring, the prepared above-mentioned solutions were mixed according to the order of calcium nitrate aqueous solution, potassium dihydrogen phosphate aqueous solution, samarium nitrate aqueous solution and urea aqueous solution.
[0035] Under stirring conditions at room temperature, ammonia water (5 mol / L) was added dropwise into the mixed solution, and the pH value of the solution was adjusted to about 10. Continue to stir the suspension for 0.5 h, and control the pH to no longer change to obtain a hydrothermal precursor. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
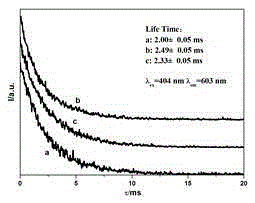
| fluorescence lifetime | aaaaa | aaaaa |
| fluorescence lifetime | aaaaa | aaaaa |
| fluorescence lifetime | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com