Prokaryotic recombinant expression and preparation method of lysyl endopeptidase
A technology of lysyl peptide chain and endopeptidase, which is applied in the field of genetic engineering and can solve the problems such as the renaturation of preL recombinantly expressed proteins.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
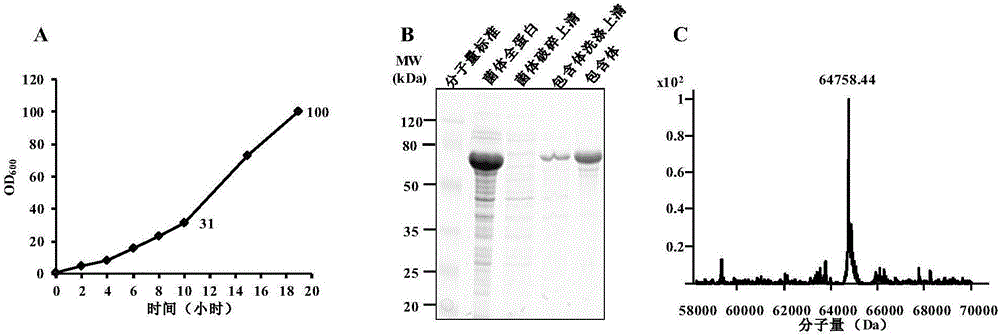
[0089] Example 1. Preparation and identification of recombinant lysyl peptide chain endopeptidase (Lys-C)
[0090] In this example, the E. coli prokaryotic expression system will be used to express recombinant lysyl peptide chain endopeptidase (Lys-C) and identify it.
[0091] 1. Preparation of recombinant E. coli expressing recombinant lysyl peptide chain endopeptidase (Lys-C)
[0092] 1. Optimization of Lysyl Peptidase (Lys-C) coding gene
[0093] The Lys-C encoding gene (GenBank number: AY062882.1, position 73-1389, GI: 17978564) derived from Pseudomonas aeruginosa, without changing the amino acid of the wild-type lysyl peptide chain Under the premise of the sequence (positions 169-606 of sequence 2), replace its codons with codons preferred by E. coli (high frequency use). The optimization also includes other modifications to the gene sequence of the peptidase within the lysyl peptide chain to be suitable for expression in E. coli, and the start codon ATG is added before the opti...
Embodiment 2
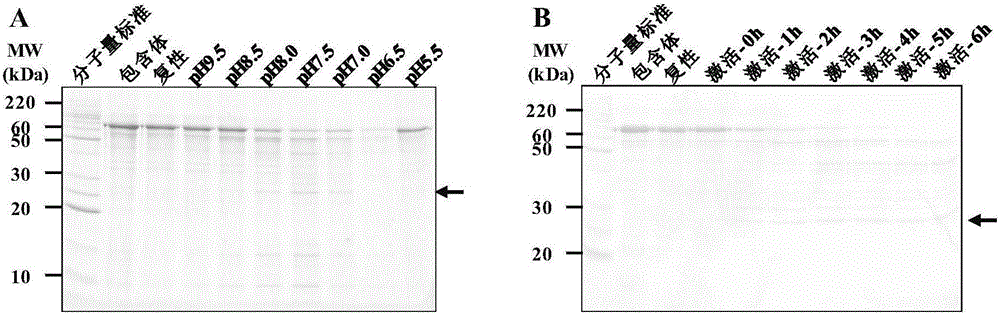
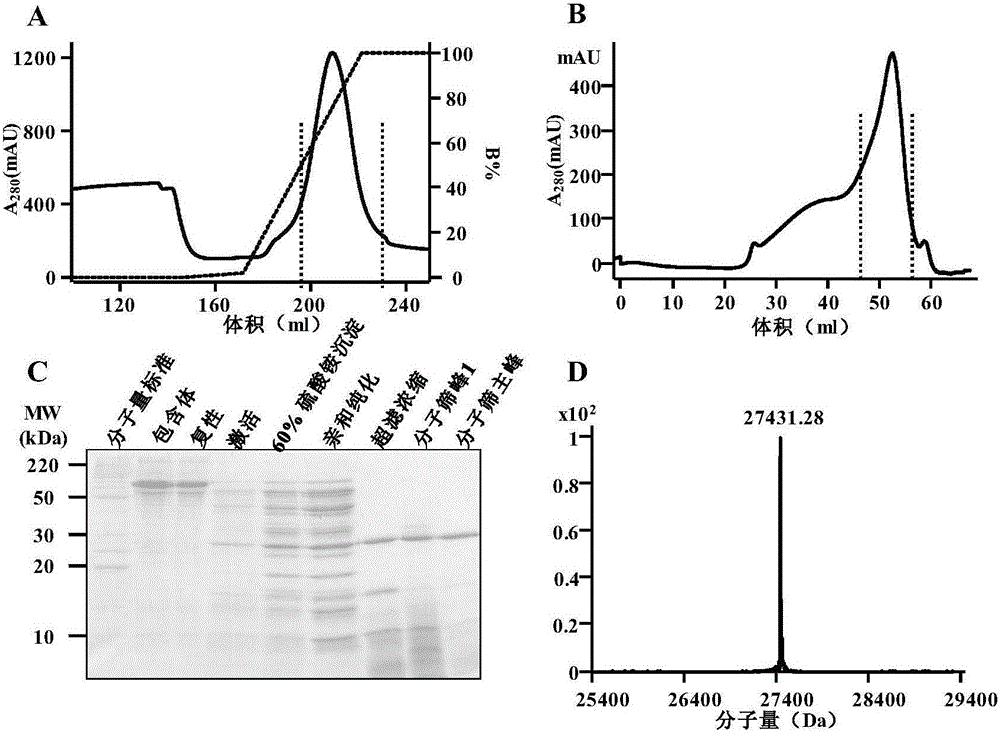
[0140] Example 2. Activity determination of recombinant lysyl peptide chain endopeptidase (Lys-C)
[0141] 1. Preparation of E. coli whole protein
[0142] E. coli BL21(DE3) (Novagen, Germany) was resuspended in PBS and then ultrasonically broken and centrifuged to obtain total bacterial protein. The protein was precipitated with pre-chilled acetone in a ratio of 1:9, and then resuspension buffer (formulation :The solvent is water; the solute and concentration are 50mM NH 4 HCO 3 And 8M urea) to resuspend, the protein concentration is 4mg / ml. Add DTT to a final concentration of 20 mM, and denature at 37°C for 45 min. After cooling the sample to room temperature, add iodized acetamide to a final concentration of 40 mM and carry out alkylation in the dark. The alkylation time is 30 min. After the alkylation is over, a 10kD ultrafiltration tube is used to change the liquid to remove DTT and acetamide iodide. Use buffer (formulation: solvent is water; solute and concentration are 50m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com