Compositions and methods for protein display on the surface of bacteria and vesicles derived therefrom and uses thereof
A protein and application technology, applied in bacteria, combinatorial chemistry, inactive components of polymer compounds, etc., can solve the problem of not carrying signal peptides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0192] Example 1 - Bacterial Strains, Plasmids and Growth Conditions
[0193] The bacterial strains and plasmids used in these examples are described in Table 1.
[0194] Table 1. Bacterial Strains and Plasmids
[0195]
[0196]
[0197]
[0198]
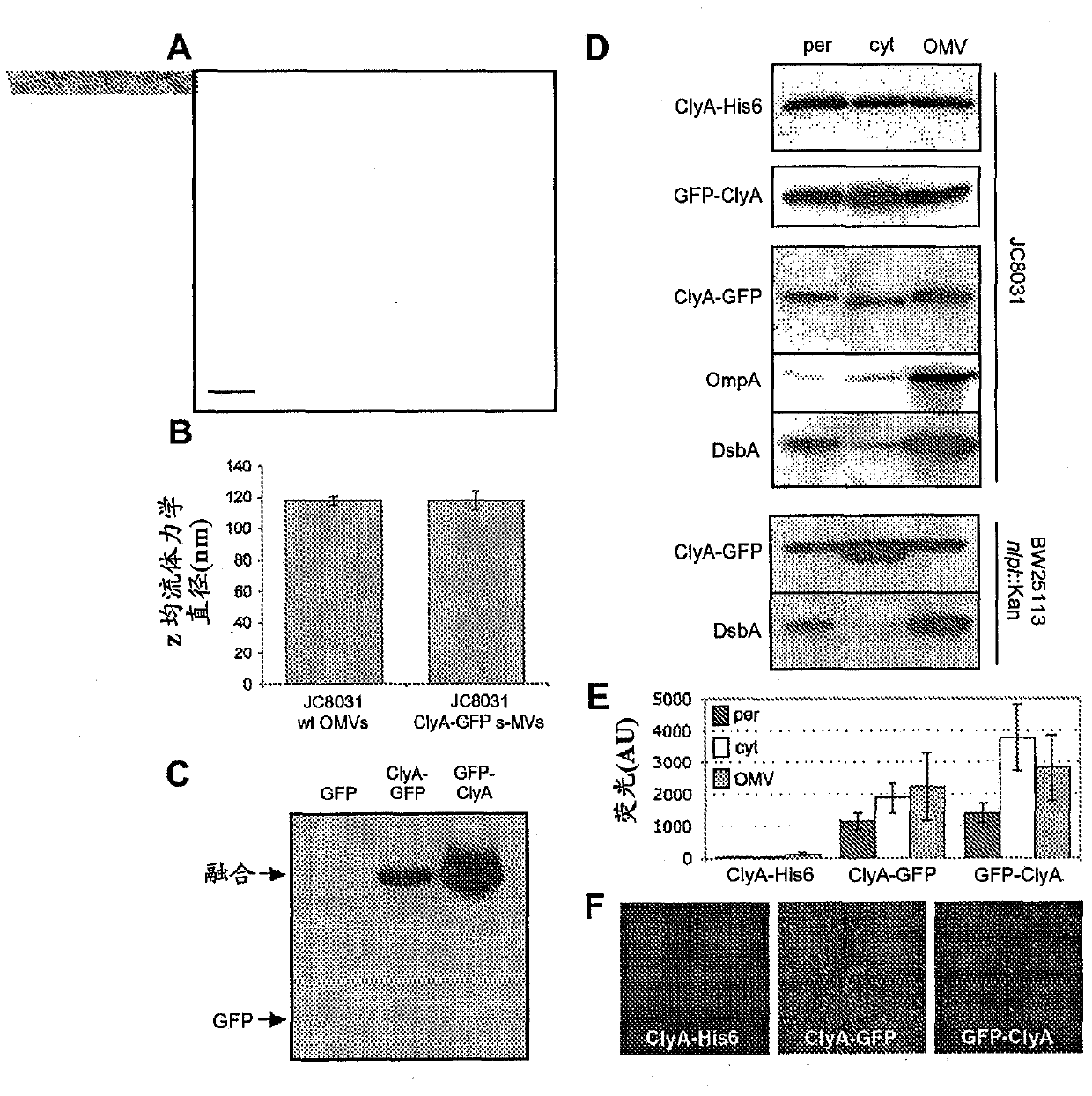
[0199]Using DHA as a donor, the dsbA::Kan allele was introduced into JC8031 via P1vir transduction to prepare cell strain JCA. The PCR-amplified clyA gene was ligated with pBAD18-Cm between SacI and XbaI sites to construct plasmid pClyA. The gene encoding gfpmut2 was inserted between the XbaI and HindIII sites respectively (Crameri et al., "Improved Green Fluorescent Protein by Molecular Evolution Using DNA Shuffling", Nat Biotechnol 14:315-9 (1996) and DeLisa et al., "Genetic Analysis of the Twin Arginine Translocator Secretion Pathway inBacteria (the genetic analysis of the Twin Arginine Translocator Secretion Pathway in bacteria)", J Biol Chem 277:29825-31 (2002), described The literature is hereby incorporated by ...
Embodiment 2
[0200] Example 2 - Cell Culture
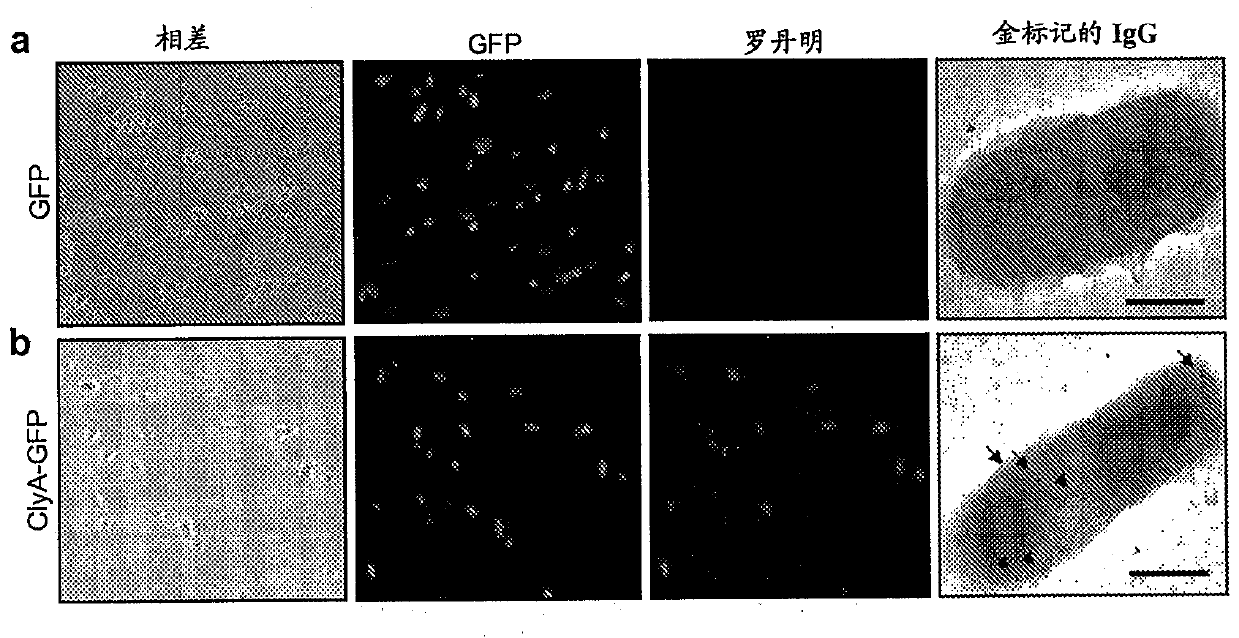
[0201] Human epithelial cervical carcinoma (HeLa) cells were obtained from the American Type Culture Collection (ATCC #CCL-2) and cultured in Dulbecco's modified Eagles supplemented with 10% NuSerum and 1% penicillin / streptomycin. Grow in minimal medium (DMEM). Maintain cells at 37°C, 95% air, 5% CO 2 in a humidified atmosphere. For fluorescence microscopy experiments, cells were grown on 12-mm round glass coverslips for 2 days prior to the experiment.
Embodiment 3
[0202] Example 3 - Subcellular Fractionation
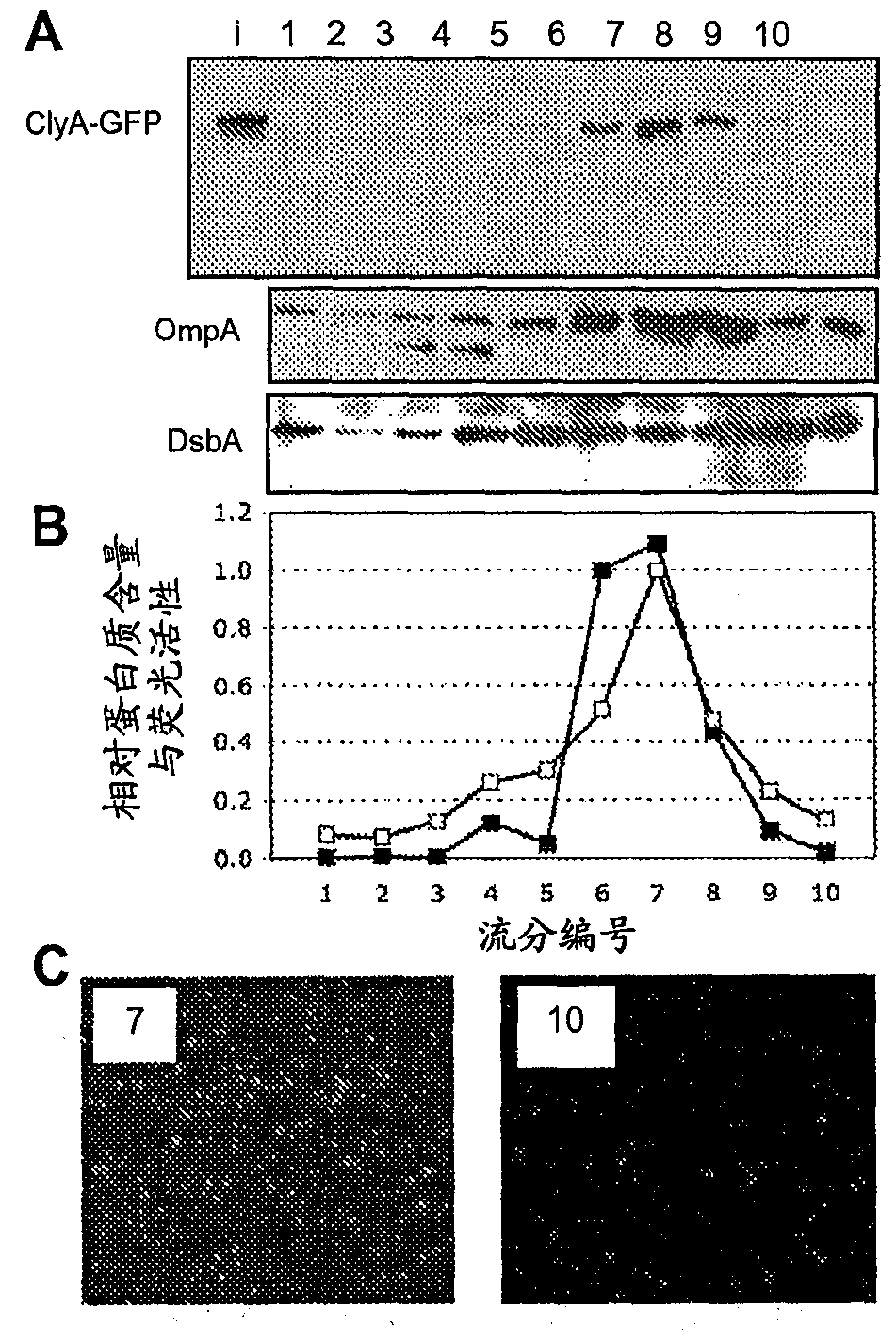
[0203] By cold osmotic shock procedure (cold osmotic shock procedure) (Kim et al., "Twin-ArginineTranslocation of Active Human Tissue Plasminogen Activator in Escherichiacoli (Twin-Arginine Translocation of Active Human Tissue Plasminogen Activator in Escherichia coli) bit)", Applied and Environmental Microbiology 71:8451-8459 (2005), which is incorporated herein by reference in its entirety), the cytoplasmic and periplasmic fractions were prepared from cells expressing the fusion protein, and the soluble fraction was removed The remaining precipitate was then collected as the insoluble fraction.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com