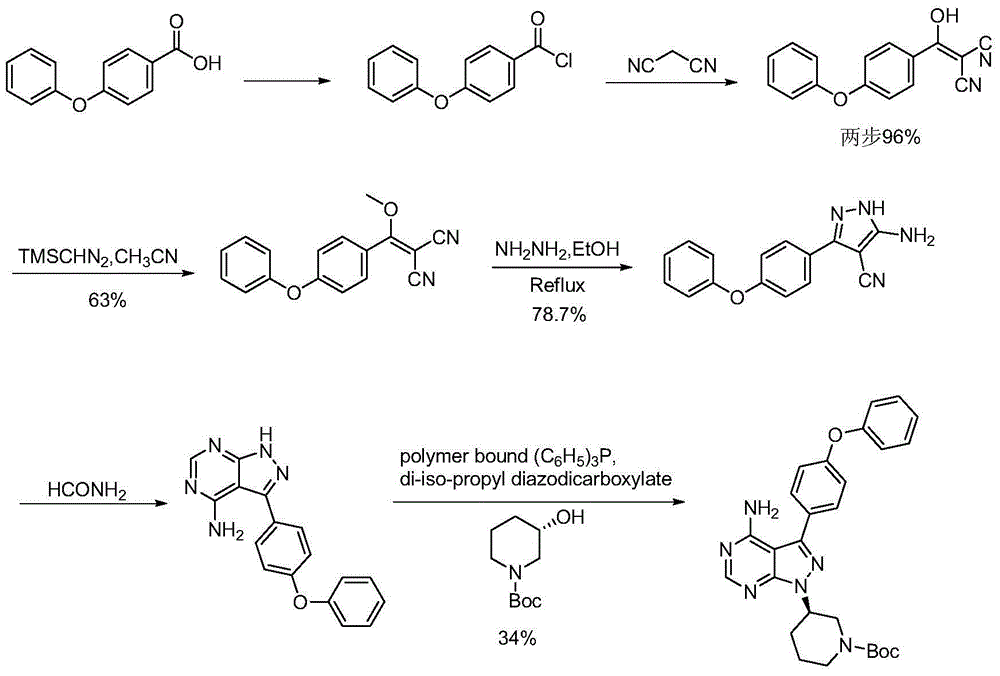
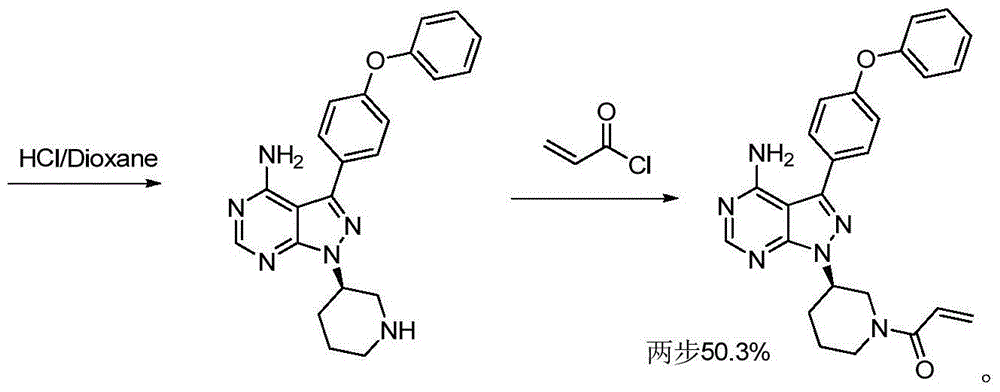
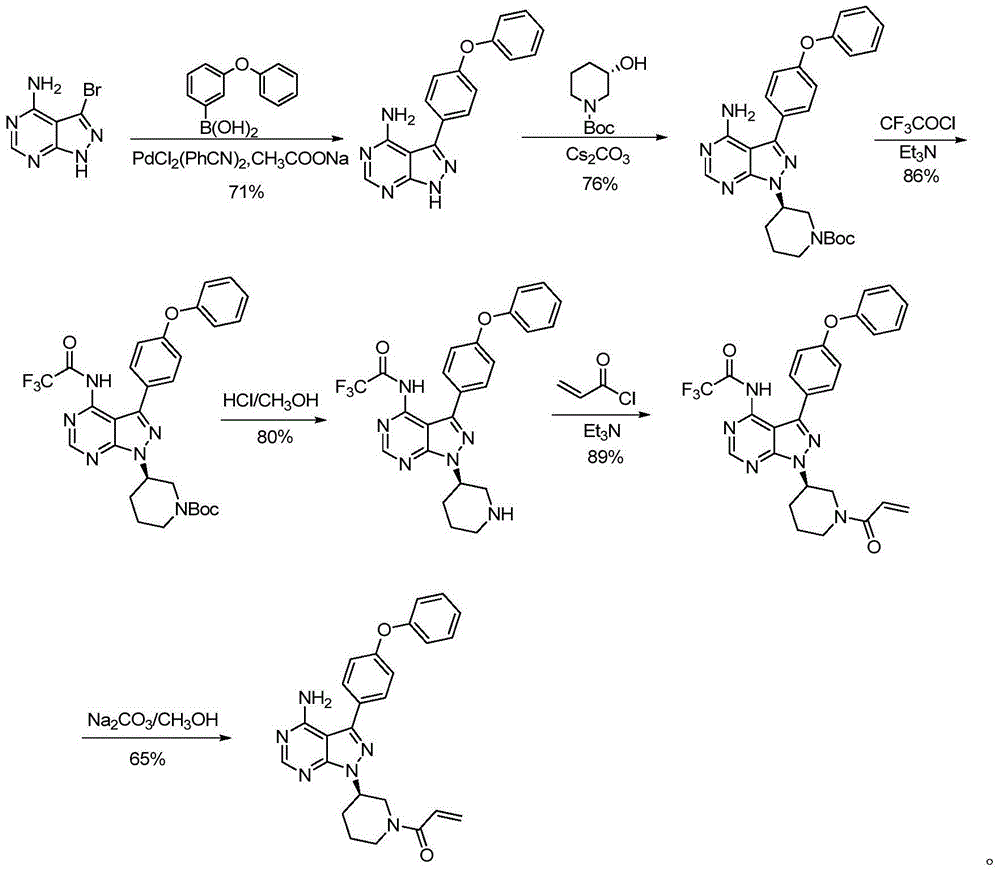
Preparation method for ibrutinib
A technology of ibrutinib and its compound, which is applied in the field of preparation of ibrutinib, can solve the problems of high cost of iodide raw materials, the yield of Mitsunobu reaction step is only 25%, and achieve the effect of high product yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050]
[0051] Under nitrogen protection, the compound of formula 1 (60g, 0.28mol), the compound of formula 2 (84.6g, 0.42mol) and triphenylphosphine (257.4g, 0.98mol) were added to anhydrous THF (10eq volume), and a light brown suspension The turbid solution was lowered to 0°C, and DIAD (198.4g, 0.98mol) was added dropwise. During the dropwise addition, the temperature was kept below 5°C, and the solution gradually turned into light yellow and clear. After dropping, it gradually rose to 20°C and stirred for 3 hours. Then, concentrated hydrochloric acid was added. (10eq), raised to 50°C and stirred for 2h, cooled to room temperature, filtered, the filter cake was washed with a small amount of THF, vacuum concentrated to dryness to constant weight to obtain 74.0g of off-white solid, yield 71.0%, chemical purity 98.5%. Take 30g and dissociate with aqueous sodium bicarbonate to obtain 22.9g of free base, with a dissociation rate of 95.1% and a chemical purity of 98.5%. m / z(MH...
Embodiment 2
[0053]
[0054] Under nitrogen protection, the compound of formula 1 (60g, 0.28mol), the compound of formula 2 (84.6g, 0.42mol) and triphenylphosphine (257.4g, 0.98mol) were added to anhydrous THF (10eq volume), and a light brown suspension The turbid liquid was lowered to 0°C, and DEAD (170.8g, 0.98mol) was added dropwise. During the dropping process, the temperature was kept below 5°C, and the solution gradually turned into light yellow and clear. After the drop, it gradually rose to 20°C and stirred for 3 hours. Then, concentrated hydrochloric acid was added. (10eq), raised to 50°C and stirred for 2h, cooled to room temperature, filtered, the filter cake was washed with a small amount of THF, and concentrated in vacuo to dryness to constant weight to obtain 70.3g of off-white solid with a yield of 67.8% and a chemical purity of 98.3%.
Embodiment 3
[0056]
[0057] Dihydrochloride (20g, 0.054mol) of the compound of formula 4, 4-phenoxyphenylboronic acid (17.35g, 0.081mol) and potassium phosphate (40.15g, 0.19mol) were dropped into 1,4-dioxane ( 200mL) and water (80mL) mixed solvent, pass through nitrogen to carry out bubbling 20min, add Pd(PPh 3 ) 4 (0.62g, 5.4×10 -4 mol), continue to feed nitrogen bubbles for 5 min, heat to reflux and stir for 5 h, the reaction solution is concentrated, ethyl acetate (100 mL) and water (100 mL) are added, the pH is adjusted to 2-3 with hydrochloric acid, liquid separation, and the aqueous phase Add ethyl acetate (100mL) for extraction once, add dichloromethane (200mL) to the aqueous phase after liquid separation, adjust the pH to 9-10 with 6N sodium hydroxide solution, stir and separate the layers, and dry the organic layer with anhydrous sodium sulfate. Evaporate to dryness to obtain 18.8 g of off-white solid, which is the free base of compound 6, with a yield of 90.0% and a chemic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com