Tolterodine sustained release microsphere preparation containing small molecular additive and preparation method thereof
A technology of tolterodine and slow-release microspheres, which is applied in the direction of medical preparations containing active ingredients, medical preparations with non-active ingredients, and pharmaceutical formulas, which can solve the problems of increased side effects, decreased drug efficacy, increased patient pain and Discomfort and other problems, to achieve the effect of reducing toxic and side effects, relieving pain, and increasing drug loading
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
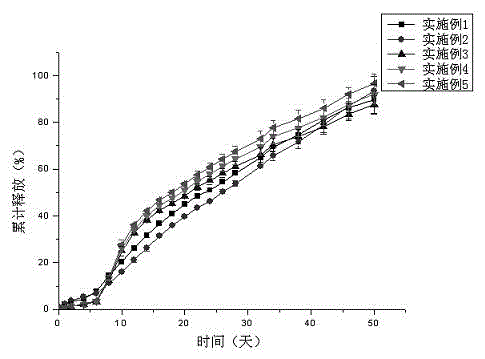
[0025] Weigh 52.9 mg of tolterodine and 300.0 mg of 7525 7E PLGA, add the above weighed substances into 0.5 ml of dichloromethane and dissolve them, and inject them into 50 ml of 0.5% PVA ( w / w) in the aqueous solution, keep the above homogeneous condition for 5 minutes, then volatile the solvent by stirring at 1000rpm for 4 hours, filter with 25μm and 125μm sieves, wash the microspheres with distilled water three times, and lyophilize. The microspheres containing 8.6% drug were prepared, the embedding rate was 57%, and the measured particle size was 50-300 μm. The line graph of the cumulative release rate of the sustained-release microspheres in the simulated release solution is shown in figure 2 .
Embodiment 2
[0027] Weigh 52.9 mg tolterodine, 300.0 mg 7525 7E PLGA, 0.3 mg stearic acid, add the above weighed substances into 0.5 ml dichloromethane to dissolve, and inject it into In 50 ml of 0.5% PVA (w / w) aqueous solution, keep the above homogeneous conditions for 5 minutes, then stir at 1000rpm to evaporate the solvent for 4 hours, filter with 25μm and 125μm sieves, wash the microspheres with distilled water three times, and freeze-dry. The microspheres containing 9.4% drug were prepared, the embedding rate was 63%, and the measured particle size was 50-300μm. The line graph of the cumulative release rate of the sustained-release microspheres in the simulated release solution is shown in figure 2 .
Embodiment 3
[0029] Weigh 52.9 mg tolterodine, 300.0 mg 7525 7E PLGA, 1.5 mg butyl stearate, add the above weighed substances into 0.5 ml dichloromethane to dissolve, and dissolve them under homogeneous (6000-8000rpm) conditions Inject into 50 ml of 0.5% PVA (w / w) aqueous solution, keep the above homogeneous condition for 5 minutes, then volatile solvent with stirring at 1000rpm for 4 hours, filter with 25μm and 125μm sieves, wash the microspheres with distilled water three times, freeze-dry . The microspheres containing 10.3% drug were prepared, the embedding rate was 68.7%, and the measured particle size was 50-300μm. The line graph of the cumulative release rate of the sustained-release microspheres in the simulated release solution is shown in figure 2 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com