Oryzanol nanocrystal capsule preparation and preparation process thereof
A nano-crystal, oryzanol technology, applied in capsule delivery, medical preparations with inactive ingredients, medical preparations containing active ingredients, etc., can solve problems such as poor bioavailability, and achieve increased solubility, improved safety, and reduced Effects of side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
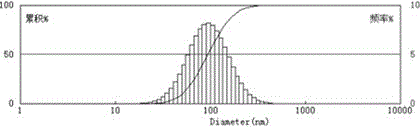
Image
Examples
Embodiment 1
[0022] Weigh 1 g of oryzanol and dissolve in 30 ml of medicinal absolute ethanol, weigh 0.1 g of PVP K30 and 0.2 g of lecithin, swell in 150 ml of water for injection, and sonicate for 10 min at room temperature. Inject the ethanol phase and the water phase at a rate of 1:5 from the inlet of the vortex mixer at a constant speed, collect at the outlet, and obtain oryzanol nano-crystal suspension.
[0023] Take the above suspension and add mannitol at a ratio of 5% (w / v), stir to dissolve, and dry it in a spray dryer at a speed of 5ml / min. The temperature is maintained at 80°C-95°C. After spray drying, collect The powder at the bottom of the drier is obtained from oryzanol nanocrystals. The obtained oryzanol nano crystals are filled into hard capsules according to the dosage of 20 mg per capsule, and then the oryzanol nano crystals capsules can be obtained.
Embodiment 2
[0025] Weigh 1 g of oryzanol and dissolve it in 30 ml of medicinal absolute ethanol, weigh 0.2 g of poloxamer F68 and 0.3 g of TPGS, swell in 150 ml of water for injection, and sonicate for 10 min at room temperature. Inject the ethanol phase and the water phase at a rate of 1:5 from the inlet of the vortex mixer at a constant speed, collect at the outlet, and obtain oryzanol nano-crystal suspension.
[0026] Take the above suspension and add trehalose at a ratio of 5% (w / v), stir to dissolve, dry in a spray dryer at a speed of 5ml / min, and maintain the temperature at 80°C-95°C. After spray drying, collect The powder at the bottom of the drier is obtained from oryzanol nanocrystals. The obtained oryzanol nano crystals are filled into hard capsules according to the dosage of 20 mg per capsule, and then the oryzanol nano crystals capsules can be obtained.
Embodiment 3
[0028] Weigh 1 g of oryzanol and dissolve it in 30 ml of medicinal absolute ethanol, weigh 0.4 g of Tween 80 and 0.2 g of TPGS, swell in 150 ml of water for injection, and sonicate for 10 min at room temperature. Inject the ethanol phase and the water phase at a rate of 1:5 from the inlet of the vortex mixer at a constant speed, collect at the outlet, and obtain oryzanol nano-crystal suspension.
[0029] Take the above suspension and add glucose at a ratio of 5% (w / v), stir to dissolve, and dry in a spray dryer at a speed of 5ml / min. The temperature is maintained at 80°C-95°C. After spray drying, collect and dry Powder at the bottom of the container to obtain oryzanol nanocrystals. The obtained oryzanol nano crystals are filled into hard capsules according to the dosage of 20 mg per capsule, and then the oryzanol nano crystals capsules can be obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com