A transdermal drug delivery system comprising rivastigmine and its preparation method
A technology for a transdermal drug delivery system and a composition, applied in the field of pharmacy, can solve the problems of high cost, complicated production process, expensive silicone pressure-sensitive adhesive and the like, and achieve the effects of low cost and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Get 12g rivastigmine tartrate, 16g polyvinylpyrrolidone K-90F, 22.4g diethylene glycol monoethyl ether, 2.8g ethyl lactate, 0.08g α-tocopherol, and 72g cosolvent into the conical flask, and use Ultrasonic instrument was used for ultrasonic dissolution for more than 4 hours to ensure that the solution was completely miscible, and 72g of acrylic pressure-sensitive adhesive DURO-TAK 87-2287 (containing 51% dry glue) was added, and stirred with a magnetic stirrer at 300 rpm for 4 hours. Until the solution is completely dissolved; let the solution in the Erlenmeyer flask stand for at least 1 hour or until all the bubbles disappear before use. Use a knife coater or hand-coat the obtained uniform glue onto a single-layer siliconized PET polyester film protective layer material (SCOTCH PAK 1022, 3M, St.Paul, US) and dry at 80°C for 30 minutes In order to remove the co-solvent, it is pressed with the backing layer (SCOTCH PAK 1109, 3M, St. Paul, US), punched, and packaged to obt...
Embodiment 2 to 3
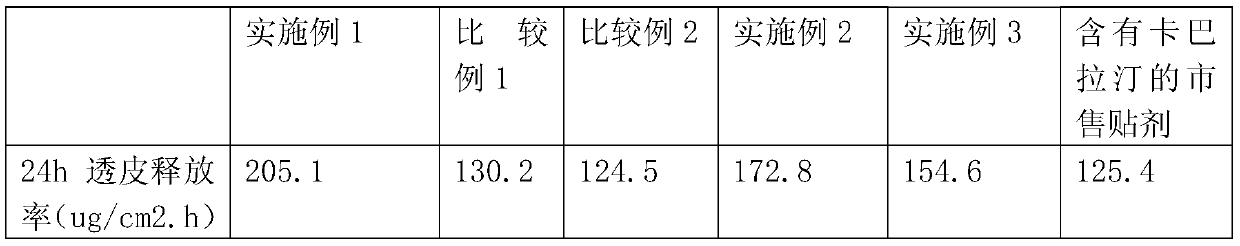
[0042] The formula composition is basically the same as in Example 1, and ethyl lactate is replaced by butyl lactate di and monolauryl lactate in Example 1 respectively. The patch was prepared according to the method of Example 1 and the transdermal release rate of rivastigmine in the permeate was determined. The results are shown in Table 1.
[0043] The results in Table 1 show that Examples 1 to 3 all show good drug permeability, and wherein Example 1 has the best effect. On the other hand, when a single penetration enhancer is used, because there is no synergistic effect, the penetration enhancement effect of rivastigmine will be significantly reduced, as shown in Comparative Examples 1 to 2 in Table 1;
[0044] Table 1:
[0045]
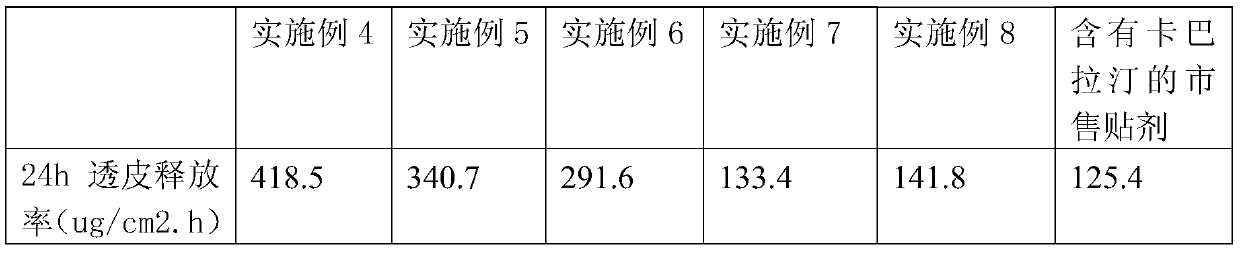
Embodiment 4
[0047] Take 24g of rivastigmine tartrate, 16g of Eudragit RL 100, 22.4g of diethylene glycol monoethyl ether, 5.6g of lauryl lactate, 0.08g of α-tocopherol, and 72g of cosolvent into a conical flask, and use an ultrasonic instrument Ultrasonic dissolving for more than 4 hours to ensure that the solution is completely miscible, add 72g of acrylic pressure-sensitive adhesive DURO-TAK 87-2287 (containing 51% dry glue), stir with a magnetic stirrer at 300 rpm for 4 hours, according to the example The patch was prepared by the method of 1 and the transdermal release rate of rivastigmine in the permeate was measured. The results are shown in Table 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com