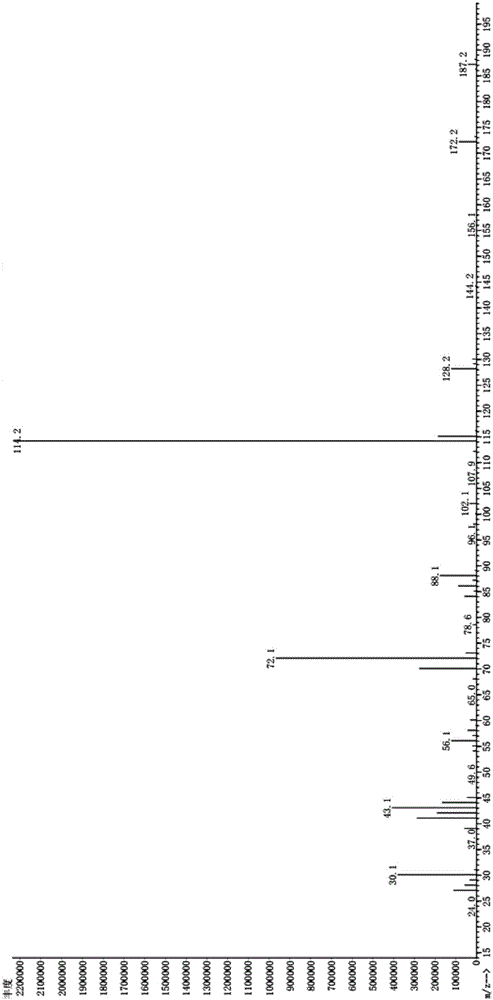
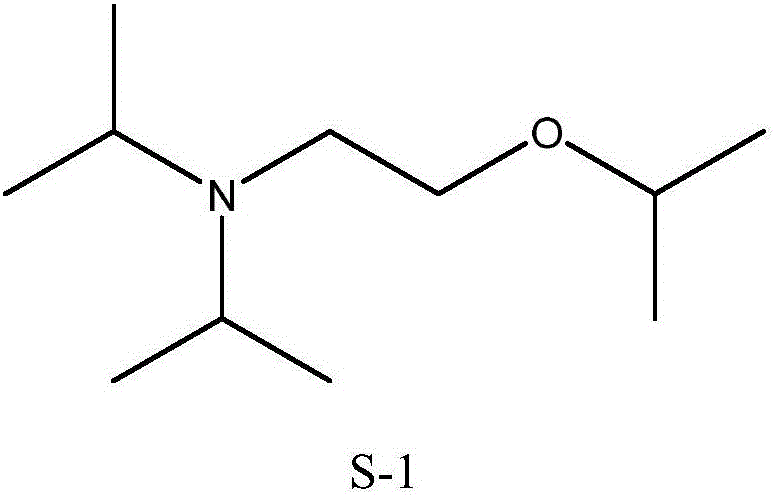
Synthesis method of N,N-diisopropyl-2-isopropoxy ethylamine
A technology of isopropoxyethylamine and diisopropyl, applied in the field of organic compound preparation, can solve the problems of complex process, high price, unsuitable for industrial production, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1, a kind of synthetic method of N,N-diisopropyl-2-isopropoxyethylamine,
[0034] Add 0.2 mol of diisopropylamine, 1.0 mol of 1,2-dichloroethane, 1.0 mol of isopropanol, and 0.3 mol of anhydrous sodium carbonate solid into the autoclave. Then close the lid of the kettle, start stirring after leak detection, heat to 180°C, and the pressure is 1.5MPa. The reaction was continued for 7 hours with no change in pressure during the process. Stop heating after the reaction time is up, stop stirring after cooling, open the kettle, pour out the reaction solution, add saturated sodium hydroxide solution, adjust the pH to 10-12, neutralize the hydrochloride in the product, and obtain an oil layer containing the target product (located in the upper layer), rectification (batch rectification under normal pressure, collecting fractions at 210-214° C.) to obtain the target product with a yield of 92.47%.
[0035] Embodiment 2-Embodiment 7, change the process parameters in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com