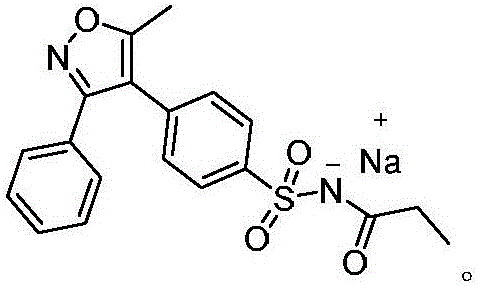
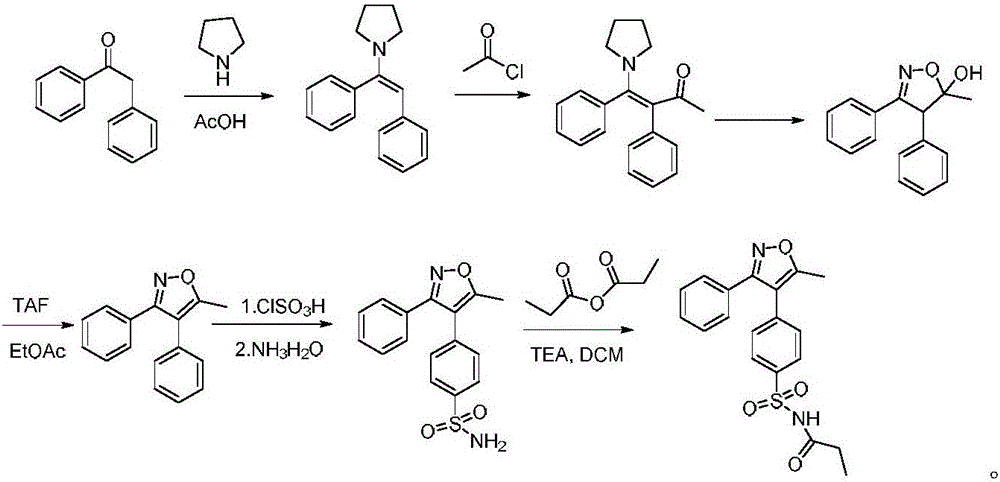
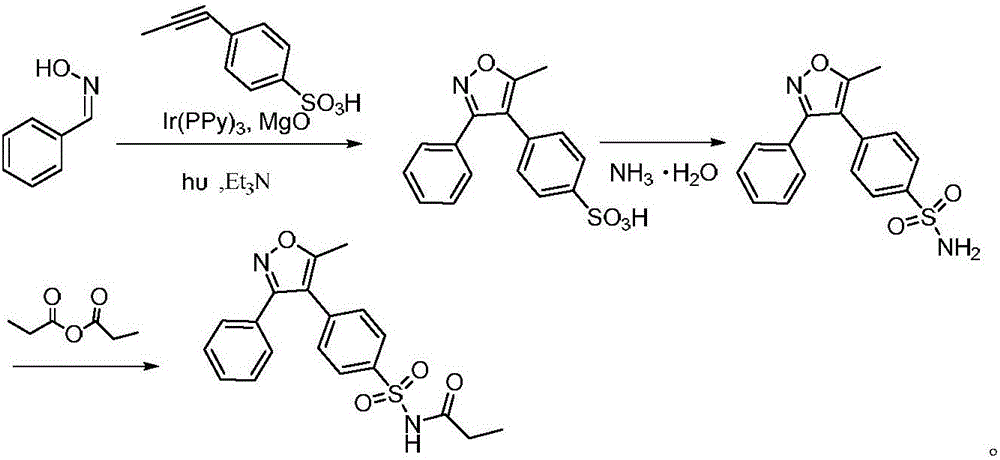
Method for preparing cyclooxygenase-2 inhibitor parecoxib
A technology of cyclooxygenase and valdecoxib, which is applied in the direction of organic chemistry, can solve the problems of waste discharge operator safety impact, compound corrosiveness, and difficult reaction treatment, so as to reduce the reaction time, increase the reaction yield, The effect of simple reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Preparation of 5-methyl-3-phenyl-4-(4-sulfophenyl)isoxazole
[0026] Mix 12.1g (100mmol) of benzaldoxime with 23.5g (120mmol) of 1-(4-sulfophenyl) propyne, 1.9g (3mmol) of tris(2-phenylpyridine) iridium(III), triethyl Add 8.1g (80mmol) of amine and 1g (25mmol) of magnesium oxide into a flask filled with 30ml THF, and carry out the light reaction at 25°C for 30min. The light is emitted by a blue light-emitting diode with a wavelength of 455nm. The reaction solution was concentrated, washed with water, recrystallized from ethanol, and dried to obtain 31.1 g of 5-methyl-3-phenyl-4-(4-sulfophenyl)isoxazole with a yield of 98.7% and a purity of 99.69%.
Embodiment 2
[0028] Preparation of 5-methyl-3-phenyl-4-(4-sulfophenyl)isoxazole
[0029] Mix 12.1g (100mmol) of benzaldoxime with 21.6g (110mmol) of 1-(4-sulfophenyl) propyne, 3.3g (5mmol) of tris(2-phenylpyridine) iridium(III), triethyl Add 8.1g (80mmol) of amine and 1.2g (30mmol) of magnesium oxide into a flask filled with 30ml THF, and carry out the light reaction at 25°C for 30min. The light is emitted by a blue light-emitting diode with a wavelength of 455nm. The reaction solution was concentrated, washed with water, recrystallized from ethanol, and dried to obtain 31.2 g of 5-methyl-3-phenyl-4-(4-sulfophenyl)isoxazole with a yield of 98.9% and a purity of 99.50%.
Embodiment 3
[0031] Preparation of 5-methyl-3-phenyl-4-(4-sulfophenyl)isoxazole
[0032] Mix 12.1g (100mmol) of benzaldoxime with 25.5g (130mmol) of 1-(4-sulfophenyl) propyne, 2.6g (4mmol) of tris(2-phenylpyridine) iridium(III), triethyl Add 7.1g (70mmol) of amine and 1g (25mmol) of magnesium oxide into a flask containing 30ml of THF, and carry out the light reaction at 30°C for 40min. The light is emitted by a blue light-emitting diode with a wavelength of 460nm. The reaction solution was concentrated, washed with water, recrystallized from ethanol, and dried to obtain 30.7 g of 5-methyl-3-phenyl-4-(4-sulfophenyl)isoxazole with a yield of 97.4% and a purity of 99.42%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com