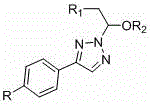
N<2> selective enol ether substituted triazole derivatives and preparation method thereof
A technology for triazole and derivatives, which is applied in the field of triazole derivatives and their preparation, can solve the problems of low yield and low selectivity, and achieve the effects of high selectivity, readily available raw materials, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The following steps are used to prepare 4-phenyl-2-(tetrahydro-2H-pyran-2-yl) 2H-1,2,3-triazole compound: under air atmosphere, add Tetrahydropyran (37.8mg, 0.45mmol), 4-phenyl-1H-1,2,3-triazole (43.5mg, 0.3mmol), iron nitrate nonahydrate (7.3mg, 0.03mmol), 10mL Ethyl chloride, stirred at 60 degrees Celsius for 10 hours, cooled to room temperature after the reaction, filtered with diatomaceous earth, extracted with water and ethyl acetate to remove the solvent, concentrated the organic layer and separated by silica gel column chromatography to obtain 55.0 mg of light yellow oily liquid , the yield was 80%. The structure of this compound is:
[0023]
[0024] Proton NMR spectrum (500MHz, CDCl 3 ): δ=7.94(s,1H),7.84–7.86(m2H),7.43–7.46(m2H),7.36–7.39(m 1H),5.78(dd,J=9.0,2.5Hz,1H),4.09–4.12 (m,1H), 3.77–3.81(m,1H), 2.50–2.53(m,1H), 2.12–2.19(m,2H), 1.66–1.80(m,3H).
[0025] C NMR spectrum (125MHz, CDCl 3 ): δ=148.08, 131.50, 130.08, 128.72, 128.50, 126.03, 89.04, 6...
Embodiment 2
[0029] Preparation of 4-methylphenyl-2-(tetrahydro-2H-pyran-2-yl) 2H-1,2,3-triazole compound adopts the following steps: Add tetrahydropyran (37.8mg, 0.45mmol), 4-methylphenyl-1H-1,2,3-triazole (47.7mg, 0.3mmol), iron nitrate nonahydrate (7.3mg, 0.03mmol) ), 10mL dichloroethane, stirred at 60 degrees Celsius for 10 hours, cooled to room temperature after the reaction, filtered with diatomaceous earth, extracted with water and ethyl acetate to remove the solvent, concentrated the organic layer and separated by silica gel column chromatography to obtain light yellow Oily liquid 56.1 mg, yield 77%. The structure of this compound is:
[0030]
[0031] Proton NMR spectrum (500MHz, CDCl 3 ): δ=7.88(s,1H),7.71(d,J=8.0Hz,2H),7.22(d,J=8.0Hz,2H),5.73(dd,J=9.0,2.5Hz,1H),4.05 –4.08(m,1H),3.73–3.78(m,1H),2.45–2.49(m,1H),2.37(s,3H),2.08–2.15(m,2H),1.62–1.75(m,3H) .
[0032] Carbon NMR spectrum (125MHz, CDCl 3 ): δ = 148.23, 138.49, 131.43, 129.51, 127.36, 126.03, 89.11, 67.49, 29.4...
Embodiment 3
[0036] Preparation of 4-(4-methoxyphenyl)-2-(tetrahydro-2H-pyran-2-yl) 2H-1,2,3-triazole compound adopts the following steps: under air atmosphere, to Add tetrahydropyran (37.8mg, 0.45mmol), 4-(4-methoxyphenyl)-1H-1,2,3-triazole (52.5mg, 0.3mmol) sequentially into a 50mL reaction flask, Ferric nitrate nonahydrate (7.3mg, 0.03mmol), 10mL dichloroethane, stirred at 60 degrees Celsius for 10 hours, cooled to room temperature after the reaction, filtered with diatomaceous earth, extracted with water and ethyl acetate to remove the solvent, and concentrated the organic layer Separated by silica gel column chromatography, 58.3 mg of light yellow oily liquid was obtained with a yield of 75%. The structure of this compound is:
[0037]
[0038] Proton NMR spectrum (500MHz, CDCl 3): δ=7.86(s,1H),7.74(d,J=8.5Hz,2H),6.97(d,J=8.5Hz,2H),5.75(dd,J=9.0,2.5Hz,1H),4.08 –4.11(m,1H),3.86(s,3H),3.76–3.81(m,1H),2.46–2.54(m,1H),2.10–2.17(m,2H),1.66–1.78(m,3H) .
[0039] C NMR spectrum (125M...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com