Application of male sterility gene OsDPW2 and rice sterility recovery method
A male sterility gene and male sterility technology, applied in the fields of application, genetic engineering, chemical instruments and methods, etc., can solve the limitation of the relationship between the restorer line and the maintainer line, the difficulty of screening excellent combinations, and the superior performance of three-line hybrid rice seeds Complicated problems, to achieve the effect of reducing labor costs and improving production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1, the method for the creation of rice male sterile lines
[0044] 1.1 Create osdpw2 rice male sterile lines by conventional breeding methods
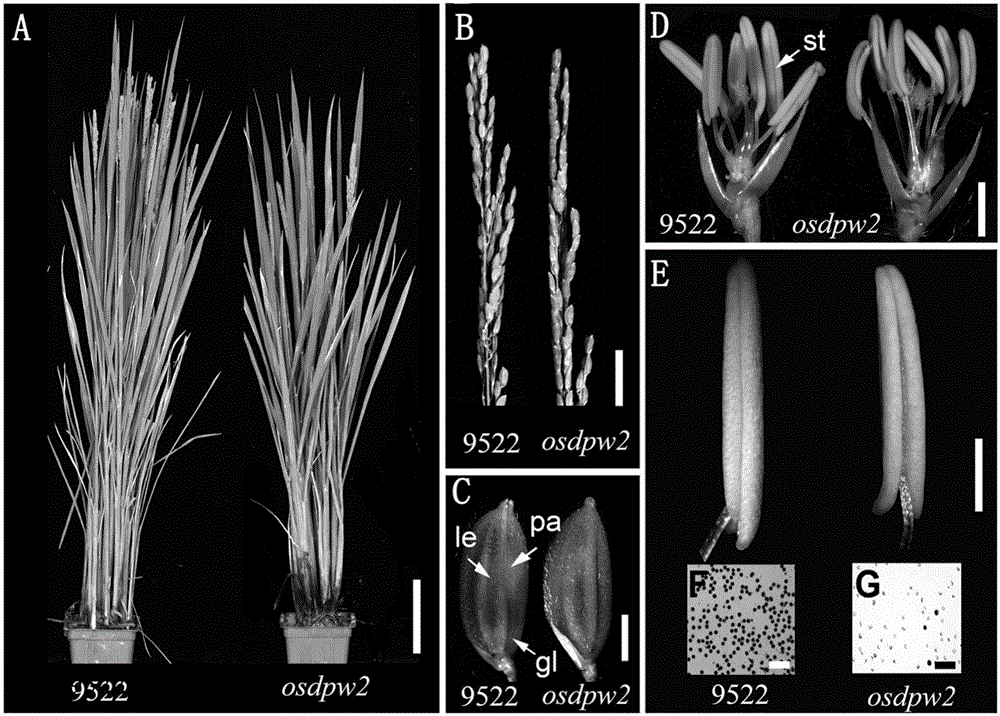
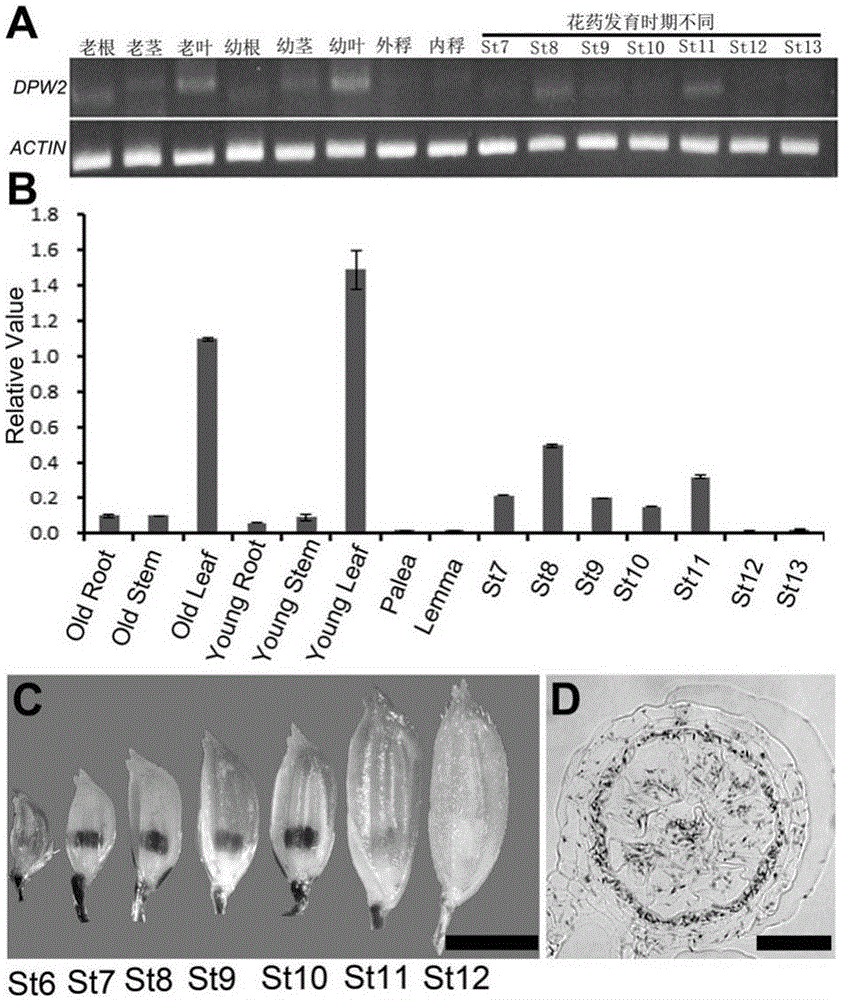
[0045] The osdpw2 mutant material in this example is obtained from conventional japonica rice variety Wuyujing No. 7 (also known as 9522) through conventional genetic engineering methods.
[0046] Those skilled in the art know that other means such as ray irradiation can also be used to induce mutations in rice conventional varieties, specifically including through 60 The osdpw2 mutant was obtained by Coγ-ray mutagenesis, and the treatment dose was 280Gy (reference method: Chen Liang, Chu Huangwei, Yuan Zheng, et al. 60Isolation and genetic analysis of rice mutants induced by Coγ-Ray radiation[J].Xiamen University Journal: Natural Science Edition, 2006, (S1): 82-85). Backcross the mutagenized mutants for three generations to obtain a stable genetic osdpw2 mutant controlled by a recessive nuclear single gene. The ...
Embodiment 2
[0067] Embodiment 2, the purposes of osdpw2 mutant in rice seed production
[0068] The osdpw2 mutant was used as the male parent to cross the sterile parent in the three-line or two-line cross combination to obtain the F1 generation. In the F2 generation, a plant with both male sterility and sterility characteristics was screened, and the plant was crossed with the maintainer line corresponding to the original sterile parent. In the F2 generation, the plants with both male sterility and sterility characteristics were screened again and crossed with the maintainer line. After multiple generations of cross screening, a new male sterile line was obtained, which was suitable as the female parent in the hybrid combination.
Embodiment 3
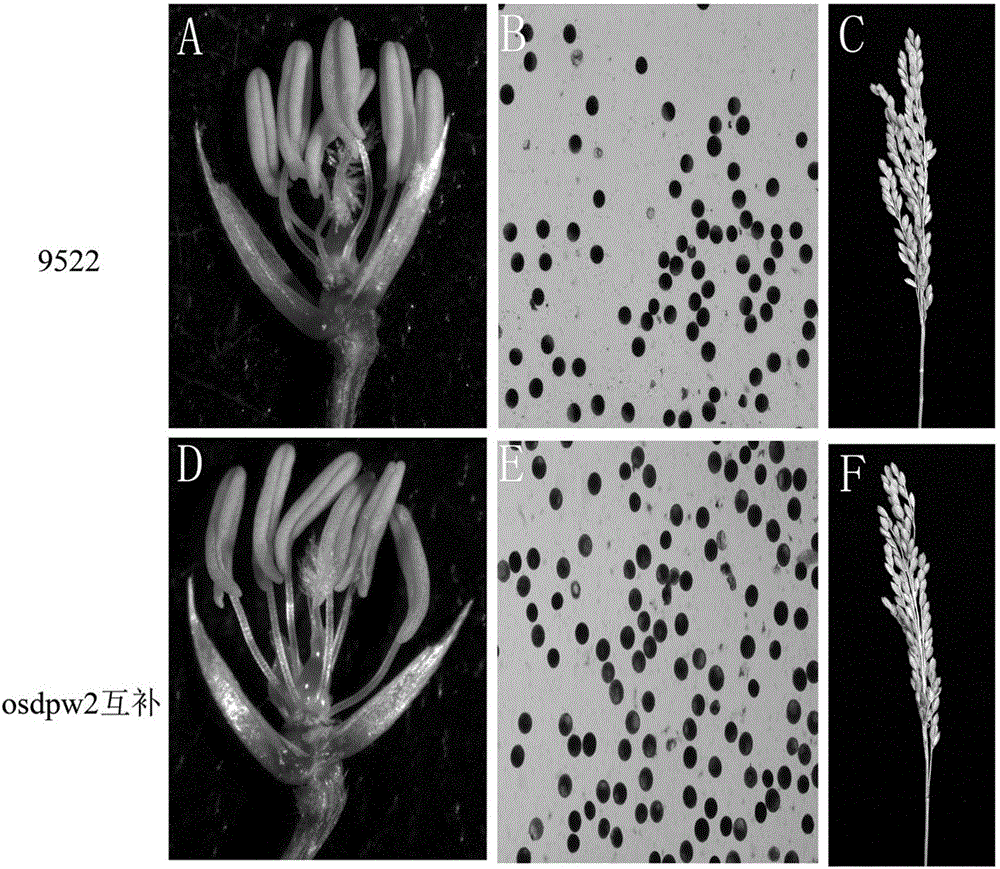
[0069] Embodiment 3, the method for recovering osdpw2 mutant male sterility traits
[0070] Transferring the genome nucleotide sequence encoding the OsDPW2 gene into the mutant osdpw2 mutant plant can restore the mutant to the wild-type phenotype.
[0071] Genomic DNA was extracted from leaves of wild-type 9522 seedlings as a template, and primers were used:
[0072] 70025gDNAF (its sequence is shown in SEQ ID NO.7):
[0073] CCATGATTAC GAATTC TATTGCCTGTTTGTGGCTGGAC,
[0074] 70025gDNAR (its sequence is shown in SEQ ID NO.8): ATTCGAGCT GGTCACC GCCGCTTACACGTTACTGTATTGG
[0075] A 5997bp genome sequence fragment of the OsDPW2 gene as shown in SEQ ID NO.6 was amplified;
[0076] Insert the amplified DNA fragment into the binary vector DCAMBIA1301 vector used for transforming rice through the In-Fusion transformation system of Dalian Bao Biology Co., Ltd.; the sequence verification is correct, and the vector is introduced into Agrobacterium tumefaciens by electroporation. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com