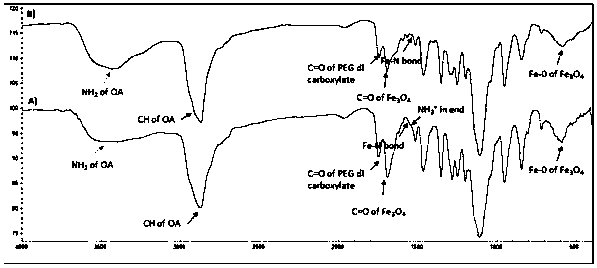
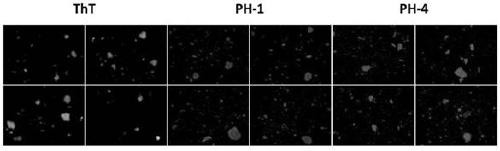
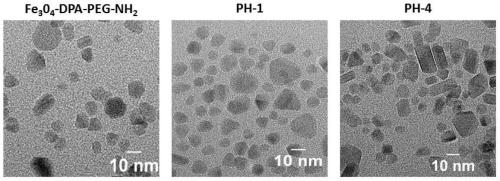
A kind of multifunctional near-infrared fluorescent magnetic nanoparticle and its preparation and application
A magnetic nano, near-infrared technology, applied in the field of biomedicine, to achieve the effect of improving efficiency and accuracy, overcoming differences in biological distribution and selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: Synthesis of 10-methyl-10H-phenothiazine-3-carbaldehyde (ie compound 2, wherein R is H)
[0040] Weigh NaH (60% purity, 1.0 g, 25.1 mmol), slowly add DMF (10 mL) in a 50 mL round bottom flask in an ice-water bath, then add methyl iodide (1.3 g, 11.04 mmol) and compound Phenothiazine (2 g, 10.0 mmol), transferred to room temperature and stirred for 2 h, TLC monitored the reaction, added water, extracted with DCM (50 ml x 3), dried over magnesium sulfate and then concentrated to dryness, separated by silica gel column to obtain compound 1 (R is H), that is, 10-methyl-10H-phenothiazine (2.1 g), white solid, melting point: 96°C, yield 97%.
[0041] 1 H NMR (400MHz, (CD 3 ) 2 CO)δ7.21(td, J=8.0,1.5Hz,2H),7.21(d,J=2.0Hz,1H),7.14(d,J=1.5Hz,1H),6.96-6.93(m,4H) ,3.39(s,3H).
[0042] Slowly add phosphorus oxychloride (1.98 g, 1.18 mL, 12.9 mmol) dropwise into a 25 mL round bottom bottle with dry DMF (904 L, 11.73 mmol) in an ice-water bath at 0°C, and stir for 0.5...
Embodiment 2
[0044] Embodiment 2: Synthesis of 8-chloro-10-methyl-10H-phenothiazine-3-formaldehyde (ie compound 2, R is Cl)
[0045] Referring to the first step of the operation process in Example 1, the product compound 1 (R is Cl), namely 2-chloro-10-methyl-10H-phenothiazine, was isolated as a white solid with a melting point of 75°C and a yield of 97%.
[0046] 1 H NMR (400MHz, CDCl 3 )δ7.16(td, J=7.8,1.3Hz,1H),7.12(dd,J=7.8,1.3Hz,1H),6.99(d,J=8.5Hz,1H),6.94(t,J=7.5 Hz,1H),6.88(dd,J=8.5,2.0Hz,1H),6.78(d,J=8.5Hz,1H),6.73(d,J=2.0Hz,1H),3.29(s,3H).
[0047] Referring to the second step of the operation in Example 1, the product 8-chloro-10-methyl-10H-phenothiazine-3-carbaldehyde was isolated as a yellow solid with a melting point of 160° C. and a yield of 38%.
[0048] 1 H NMR (400MHz, CDCl 3 )δ7.16(td, J=7.8,1.4Hz,1H),7.11(dd,J=7.8,1.4Hz,1H),7.01(d,J=8.2Hz,1H),6.93(td,J=7.6 ,1.4Hz,1H),6.88(dd,J=8.2,2.0Hz,1H),6.8(d,J=8.2Hz,1H),6.75(d,J=2.0Hz,1H),3.35(s,3H ).
Embodiment 3
[0049] Example 3: Synthesis of (E)-3-(10-methyl-10H-phenothiazin-3-yl)acrolein (i.e. compound 3, R is H)
[0050] Saturated potassium carbonate solution (15 mL) was added to tris(3,6-dioxaheptyl)amine (TDA-1) (738 mg, 2.28 mmol, 1.1 equiv) in DCM (10 mL), Then (1,3-dioxolan-2-yl)methyltriphenylphosphine bromide (1.43 g, 3.32 mmol, 1.6 equiv) and compound 2 (500 mg, 2.07 mmol, 1 equiv ). The reaction system was heated to reflux and stirred for about 20 hours. After the reaction was monitored by TLC, it was first extracted with (DCM 2×25 ml), washed with saline, and the organic phase was dried with magnesium sulfate and concentrated to dryness. Added 20 ml of THF and 10% HCl (20 mL), stirred at room temperature for 1 h. The reaction system was adjusted to pH 7 with 10% NaOH in an ice-water bath at 0 degrees Celsius, then extracted with DCM (2x25 ml) and water, finally dried with saturated brine and magnesium sulfate, concentrated to dryness, and separated by silica gel column ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com