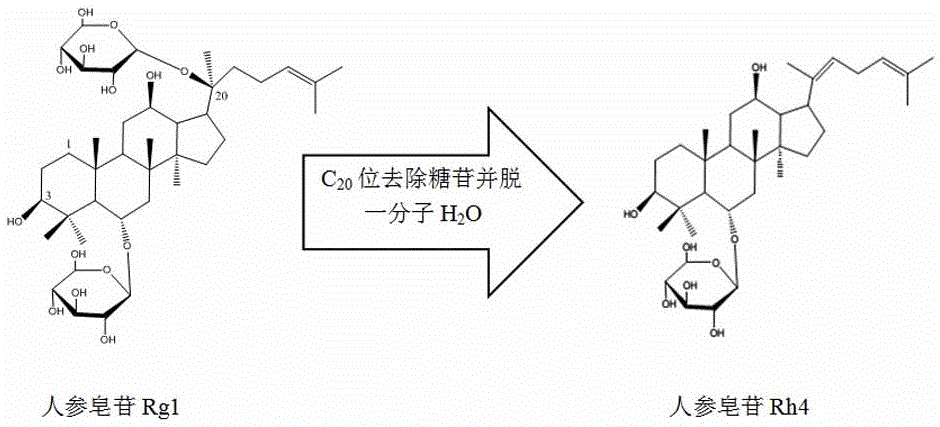
Method for producing ginsenoside Rh4 by utilizing panaxatriol ginsenoside through large-scale conversion
A large-scale technology of ginsenoside, applied in chemical instruments and methods, organic chemistry, glycoside steroids, etc., can solve the problems of inability to realize large-scale production, strict operation requirements, complicated process, etc., to improve market competitiveness, refine and purify The effect of simple process and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
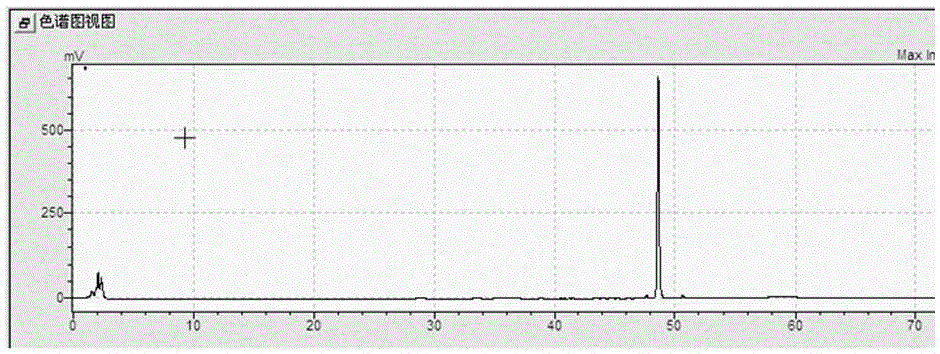
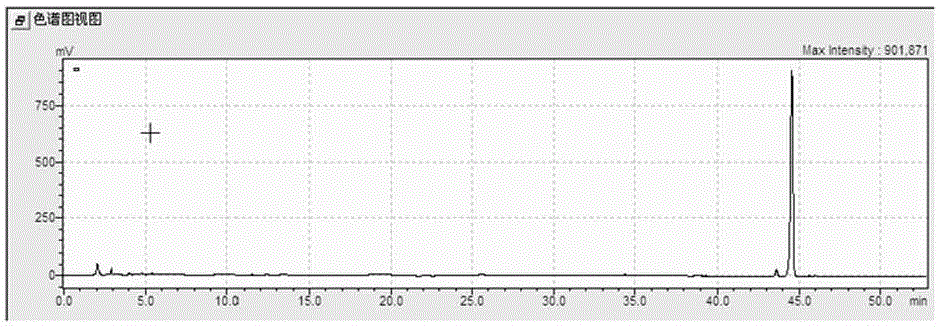
[0020] Add 65L of deionized water to a fully automatic fermenter with an effective volume of 100L, then add 3500g of ginsenoside Rg1 with a purity of 85% (impurities are other types of ginsenosides and polysaccharides), pass N 2 , the ventilation flow rate is 50 L / min, and lasts for 5 minutes, then stops the ventilation and starts the heating-in-line sterilization, and the sterilization condition is 121°C, and maintains for 20 minutes. After cooling down to below 90°C, add 5 L of sterile citric acid solution with a concentration of 0.15 mol / L and 10 g of heteropoly acid H 3 PW 12 o 40 ·5H 2 0, then heat the liquid in the fermenter to 90°C and keep it for 48h, while keeping the stirring speed at 200rpm, put all the liquid out of the container and let it stand for 12h after the reaction, collect the supernatant 20L and dry it to obtain the crude product of ginsenoside Rh4 about 3120g, the purity of ginsenoside Rh4 detected by HPLC is 92%.
Embodiment 2
[0023] Add 300L of deionized water to a fully automatic fermenter with an effective volume of 500L, and then add 90kg of ginsenoside Rg2 with a purity of 95% (the impurities are other types of ginsenosides and polysaccharides), pass N 2 , the ventilation flow rate is 350 L / min, and lasts for 4 minutes, and then the ventilation is stopped to start the online sterilization, and the sterilization condition is 121°C, and it is maintained for 20 minutes. After cooling down to below 90°C, add 20L of sterile malic acid solution with a concentration of 0.8mol / L, 10L of sterile succinic acid solution with a concentration of 0.6mol / L and 20g of heteropoly acid H 4 SiW 12 o 40 25H 2 0, then heat the fermenter to 75°C and keep it for 36 hours while keeping the stirring speed at 100rpm. After the reaction, all the liquid is released and left in the container for 24 hours, centrifuged to obtain 100L of supernatant, and there is about 120L of solid-liquid mixture at the bottom. Add 120L o...
Embodiment 3
[0025] Add 120L of deionized water to a fully automatic fermenter with an effective volume of 200L, then add 28kg of ginsenoside Rf with a purity of 90% (the impurities are other types of ginsenosides and polysaccharides), pass N 2 , the ventilation flow rate is 200 L / min, and lasts for 3 minutes, then stops the ventilation and starts the online sterilization, the sterilization condition is 121°C, and maintains for 20 minutes. After cooling down to below 90°C, add 10L of sterile malic acid solution with a concentration of 0.3mol / L, 10L of α-ketoglutaric acid solution with a concentration of 0.25mol / L, 225mL of lactic acid and 30g of heteropoly acid H 3 FeW 12 o 40 ·30H 2 O, then heat the fermenter to 90°C and keep it for 48 hours, while keeping the stirring speed at 300rpm. After the reaction is over, release all the liquid and let it stand in the container for 24 hours. Take 60L of the supernatant, and centrifuge about 40L of the liquid containing the precipitate at the rem...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com