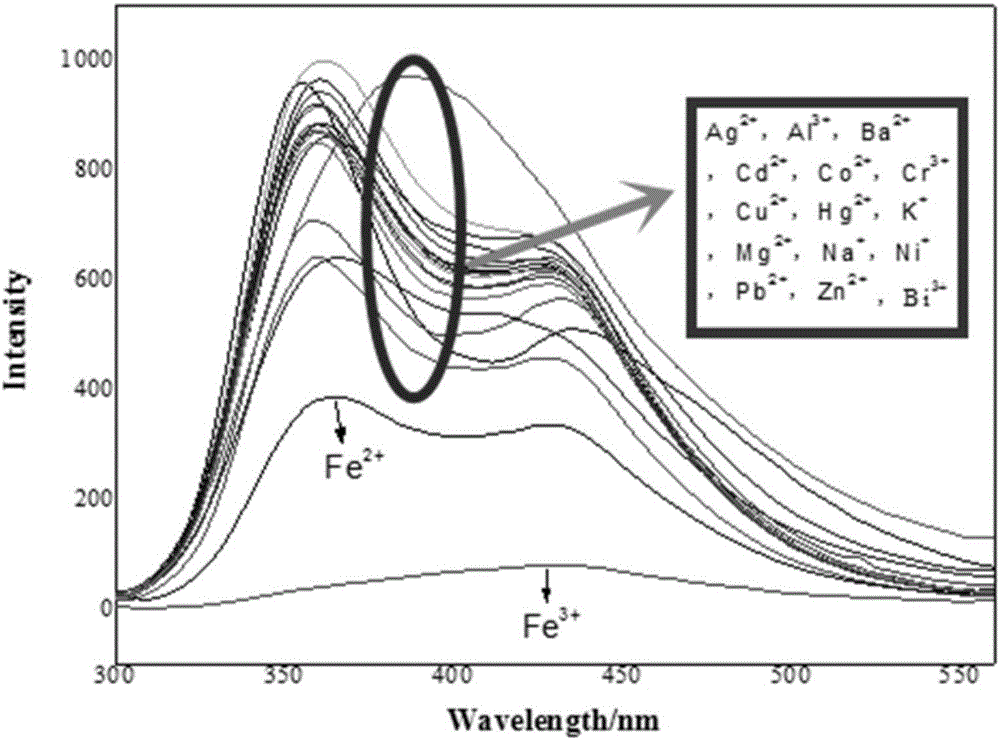
Iron ion fluorescent probe compound as well as preparation method and application thereof
A technology of fluorescent probes and compounds, applied in the field of iron ion fluorescent probe compounds and their preparation, can solve the problems affecting the accuracy of results, tedious experimental process, low water solubility, etc., achieve low cost, simple pretreatment, and synthetic method simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
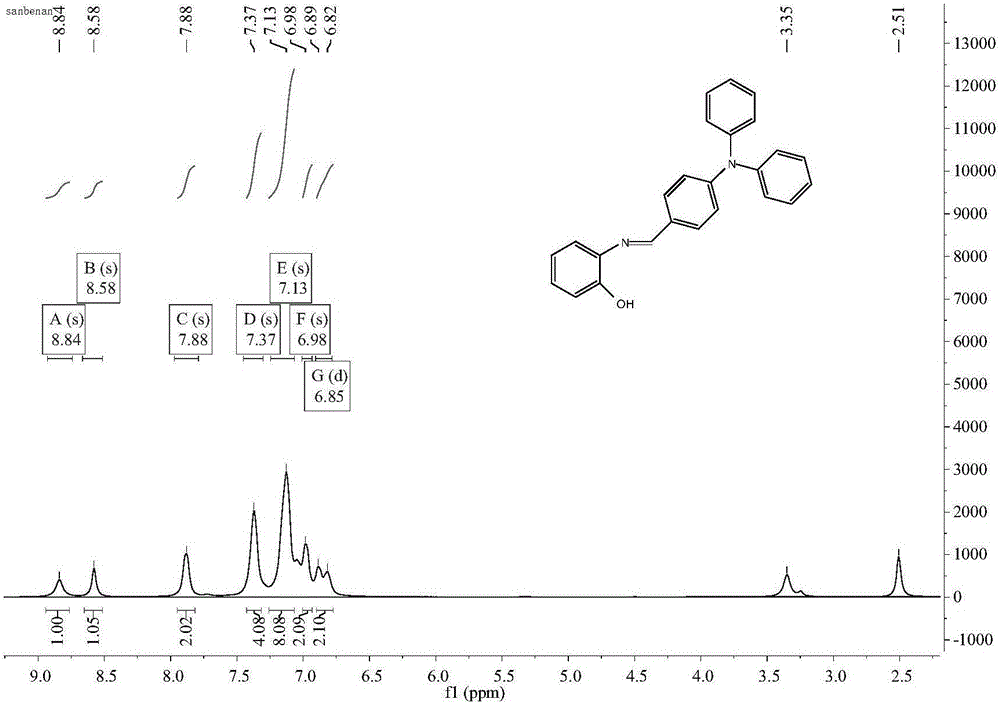
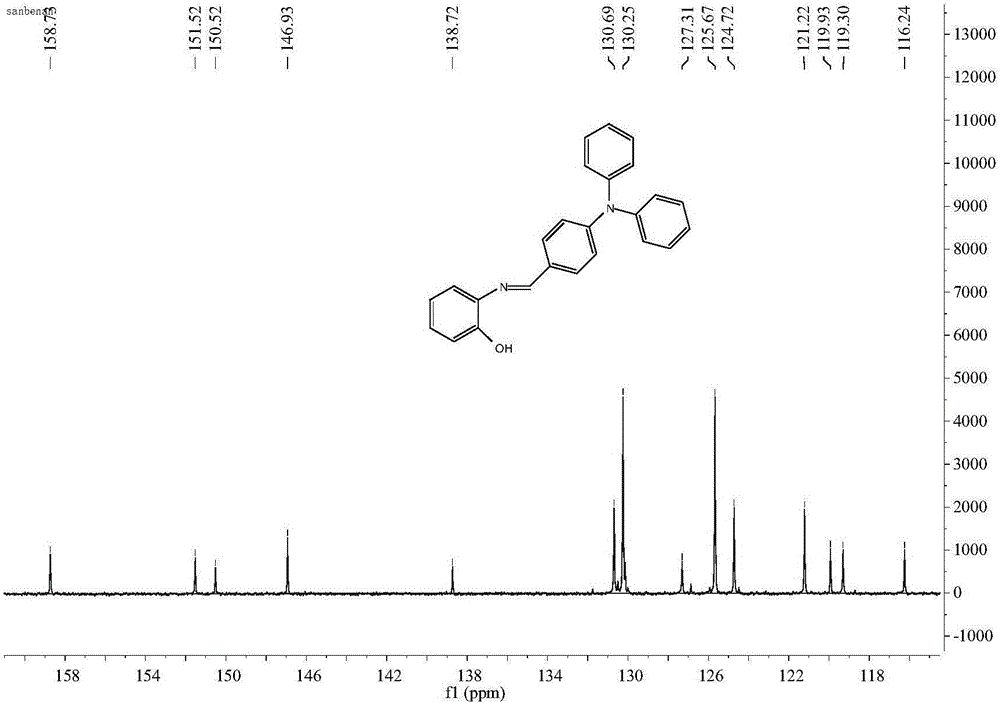
[0040] Dissolve o-aminophenol (242.0mg, 2.2mmol) and 4-diphenylaminobenzaldehyde (728.9mg, 2.0mmol) in 20ml of methanol, reflux at 80°C, fully stir at 80°C for 10h to react; after the reaction ( Determine whether the reaction takes place completely by thin-layer chromatography analysis, the analysis method is as follows) the crude product is filtered, and the filtrate is evaporated to remove the solvent by vacuum distillation to obtain a light brown oily residue, and the oily residue is cleaned with n-hexane. Then, a brown solid was obtained by filtration, and finally dried. The purity of the product was 100%, and the yield was 86%.
[0041] The analysis method to determine whether the reaction is complete by thin-layer chromatography: first prepare a mixed solution of ethyl acetate and petroleum ether with a volume ratio of 1:4 as a developer, use a capillary to suck a little of the reacted mixture, and then spot it on a silica gel plate , and then put the silica gel plate in...
Embodiment 2
[0043] Dissolve o-aminophenol (220.0mg, 2.0mmol) and 4-diphenylaminobenzaldehyde (728.9mg, 2.0mmol) in 20ml of methanol, reflux at 85°C, fully stir at 80°C for 10h to react; after the reaction ( Determine whether the reaction occurs completely by thin-layer chromatography analysis, and the analysis method is the same as in Example 1) The crude product is filtered, and the filtrate is evaporated to remove the solvent by vacuum distillation to obtain a light brown oily residue, and the oily residue is cleaned with n-hexane , and then filtered to obtain a brown solid, and finally dried, the purity of the product was 100%, and the yield was 83%.
Embodiment 3
[0045] Dissolve o-aminophenol (286.0mg, 2.6mmol) and 4-diphenylaminobenzaldehyde (728.9mg, 2.0mmol) in 20ml of methanol, reflux at 90°C, fully stir at 80°C for 10h to react; after the reaction ( Determine whether the reaction occurs completely by thin-layer chromatography analysis, and the analysis method is the same as in Example 1) The crude product is filtered, and the filtrate is evaporated to remove the solvent by vacuum distillation to obtain a light brown oily residue, and the oily residue is cleaned with n-hexane , and then filtered to obtain a brown solid, and finally dried, the purity of the product was 100%, and the yield was 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com