Stabilizing agent for repairing heavy metal pollution and preparation method thereof
A heavy metal stabilizer, stabilizer technology, applied in the field of heavy metal pollution control, can solve the problems of less dosage, increased pollutant capacity, secondary pollution, etc., achieves a wide range of applications, reduces the impact on the environment, and does not have secondary pollution. The effect of pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0016] In view of this, an embodiment of the present invention provides a method for preparing a stabilizer for repairing heavy metal pollution, comprising the following steps:
[0017] S01. Corn starch is provided, the corn starch is added to an alkaline solution to form a corn starch paste, the corn starch paste is subjected to the first heat treatment, and the corn starch paste containing epoxy is added dropwise during the heating process. The alkaline solution of chloropropane is subjected to the first reaction at 20-40°C, and the cross-linked starch is obtained after the first purification treatment, wherein the water content of the corn starch is ≤15%, and the alkaline solution is The pH of the mixed solution formed by sodium hydroxide and sodium chloride is 9.0-11.0, and the temperature of the first heat treatment is 20-40°C;
[0018] S02. After soaking the cross-linked starch with water, adjust the pH to 9.0-12.0 with an alkaline compound, perform a second heat treatme...
Embodiment 1
[0032] A preparation method for a stabilizer for repairing heavy metal pollution, comprising the following steps:
[0033] S11. take by weighing 100g absolute dry cornstarch, join in 150ml alkaline solution under stirring (alkaline solution is to be prepared by 500ml water dissolving prescribed sodium hydroxide 1.32g and 15g sodium chloride); Then place in 55 In a constant temperature water bath at ℃, keep stirring, add 50ml of alkaline solution (containing 0.4ml of epichlorohydrin required amount) dropwise within 3 to 5min, and keep stirring and reacting at 25℃ for 4 hours; after the reaction is completed, use 30mol / L sulfuric acid solution to adjust the pH value of the solution to 6.0-7.0, let it stand for a period of time, filter, wash, filter with suction, and dry at 55°C to obtain the sample.
[0034] S12. Put the cross-linked starch prepared above into a three-necked flask, add an appropriate amount of distilled water to moisten and submerge, and control the pH of the wh...
Embodiment 2
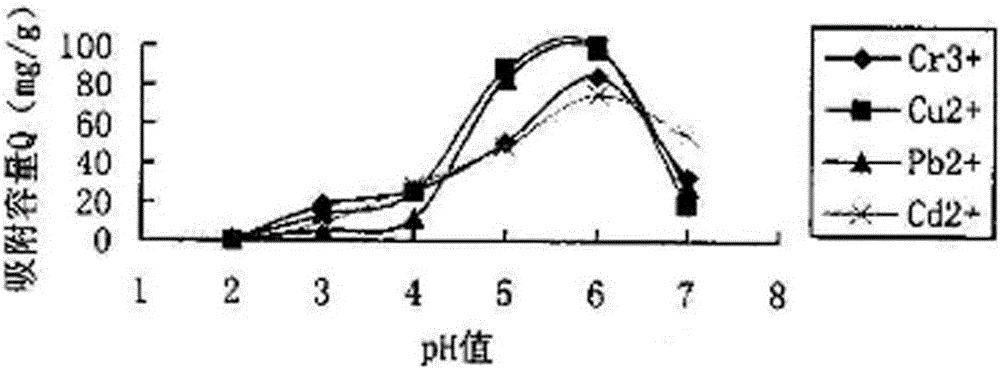
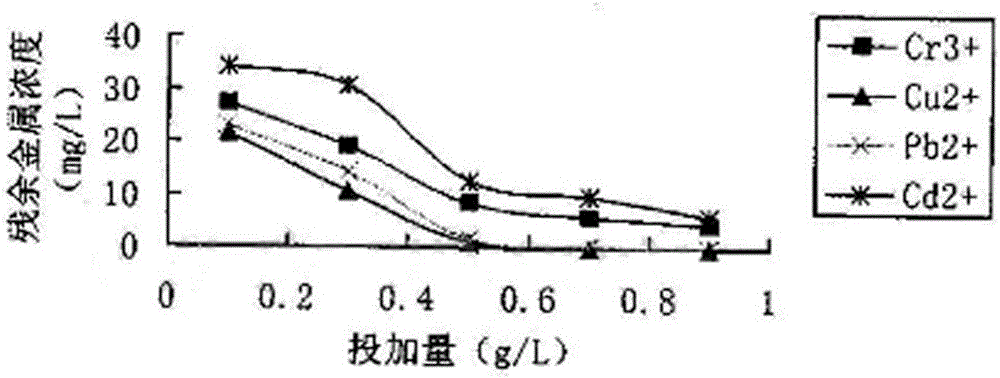
[0036] Take 100ml of 50ppm Cr 3+ 、Cu 2+ , Pb 2+ 、Cd 2+ Four kinds of standard solutions of different pH values are placed in 250ml Erlenmeyer flasks, respectively add the heavy metal polymer stabilizer prepared in the above-mentioned embodiment 1 of equal amount in every kind of standard solution, fully vibrate for a period of time, then let stand for a certain period of time. Filter after time. The concentration of residual metals in the clear night was determined by spectrophotometry and atomic absorption to determine the amount of adsorption. Calculated as follows:
[0037] Adsorption capacity Q=V×(C 0 -C) / W
[0038] In the above formula: Q=adsorption capacity; V=metal ion solution volume (L); C 0 =concentration of heavy metal ions in solution before adsorption (mg / L); C=concentration of heavy metal ions in solution after adsorption (mg / L); W=dry weight of medicament (g).
[0039] The stabilizer adsorption capacity curve figure that the embodiment of the present i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com