Method for efficiently synthesizing L-theanine through recombined corynebacterium crenatum
A technology of corynebacterium bacilli and theanine, applied in the fields of genetic engineering and enzyme engineering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Cloning of γ-glutamyl transpeptidase gene and construction of its expression vector
[0038] Using the pXMJ19 vector as a template, PrimerSTAR HS high-fidelity enzyme was selected, and two pairs of primers Nde I R, TacM-F, Nco I F, and tacM-R were used to amplify the tac-lacIq-Nde I fragment and the Nco I-oripUC-tac fragment respectively. The sizes of the gene fragments are about 2700bp and 2100bp respectively, and the overlapping sequence of the two fragments is ATTAATCATCG TGTGGTACCAT (The underlined part is the mutated site of the tac promoter and its mutated sequence). The above two DNA fragments were recovered from the gel and prepared for the next round of PCR. The PCR system includes dNTP, Pfu enzyme and its buffer, and the above two PCR products are added to make them serve as templates and primers (the total amount is set to 1 μL, and the dosage ratio can be adjusted according to the concentration of the PCR product). At a lower annealing temperatur...
Embodiment 2
[0041] Example 2: Construction of C. glutamicum SDNN403 recombinant bacteria and analysis of GGT protein expression
[0042] The recombinant plasmids pXMJ19-△sp ggt, pXMJ19-ggt, pXMJ19-tacM were transformed into C. glutamicum SDNN403 competent, and the positive recombinants C. glutamicum SDNN403 / pXMJ19-△sp ggt, C. glutamicum SDNN403 / pXMJ19-ggt were obtained by plasmid validation and screening , C. glutamicum SDNN403 / pXMJ19-tacM-ggt. Inoculate it in basal medium, cultivate overnight at 30°C, transfer to high-arginine-producing medium with 10% inoculum the next day, and add IPTG to a final concentration of 0.8mM in mid-logarithmic phase to induce expression , cultivated for 96h. After the culture, the supernatant was collected as the extracellular enzyme solution and stored at 4°C. The broken cell pellet was also reserved for subsequent analysis. The experimental results showed that only intracellular protein bands were observed in recombinant bacteria C.glutamicum SDNN403 / pX...
Embodiment 3
[0043] Embodiment 3: Recombinant bacterial strain GGT enzyme activity assay
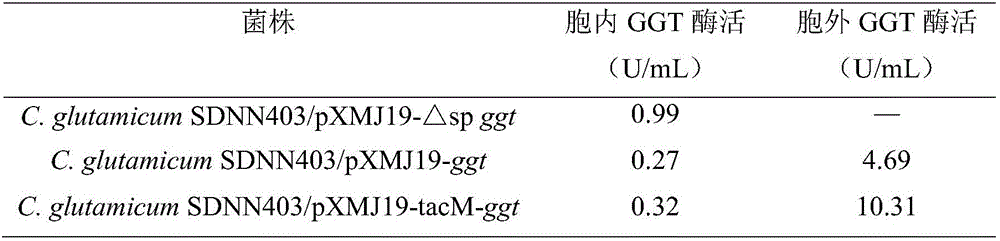
[0044]After the recombinant bacteria C. glutamicum SDNN403 / pXMJ19-△sp ggt, C. glutamicum SDNN403 / pXMJ19-ggt and C. glutamicum SDNN403 / pXMJ19-tacM-ggt were cultured in the arginine high-yield medium, the intracellular fragmentation The enzyme activity of GGT in the supernatant and supernatant of the supernatant is shown in Table 1. Since the protein content inside and outside the cell is not comparable, only absolute enzyme activity units are considered here. In the recombinant strain C. glutamicum SDNN403 / pXMJ19-△sp ggt, only 0.99 U / mL of intracellular GGT activity was detected. Enzyme activity was detected both inside and outside the cell in the recombinant strain C.glutamicum SDNN403 / pXMJ19-ggt, which were 0.27U / mL and 4.69U / mL respectively. They are 0.32U / mL and 10.31U / mL respectively. It can be seen from the enzyme activity data (Table 1) that in the recombinant bacteria C. glutamicum SDNN403 / ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com