Magnesium-based composite hydrogen storage material and preparation method thereof
A technology of hydrogen storage materials and alloys, which is applied in the direction of electrical components, circuits, battery electrodes, etc., can solve problems such as the existence of the preparation process, poor kinetics and corrosion resistance, and potential safety hazards, so as to reduce oxidation, ensure accuracy, and alloy composition Accurate and even effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
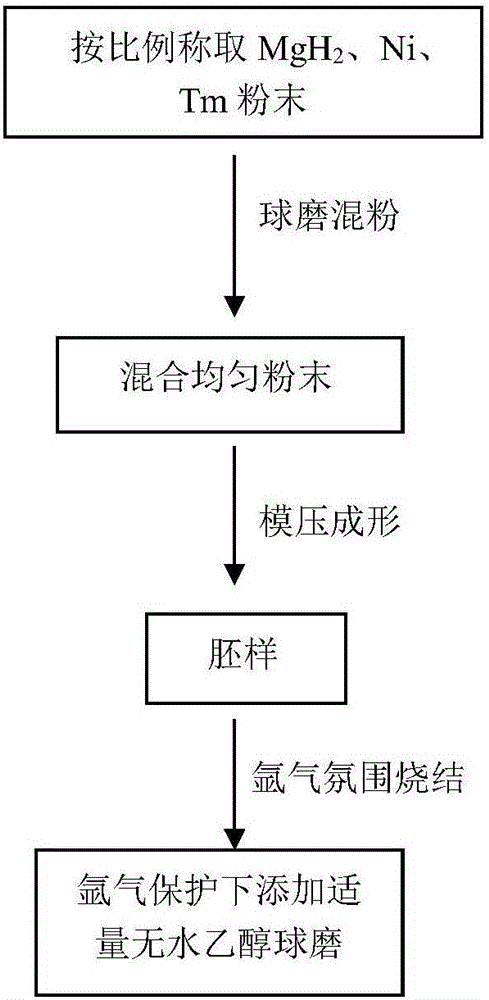
[0040] Preparation of magnesium-based composite hydrogen storage materials, the process is as follows figure 1 , the MgH 2 , Ni and Mn are mixed according to the ratio of 20:7:3 (molar ratio), using a planetary ball mill, the speed of the ball mill is set to 100 rpm, and the powder is mixed under the protection of high-purity argon, and the time of mixing is set For 5 hours, mixed powder B was obtained;
[0041] The mixed powder B is pressed into shape by using a stainless steel mold, and the pressing pressure is 60-120MPa to obtain the blank C;
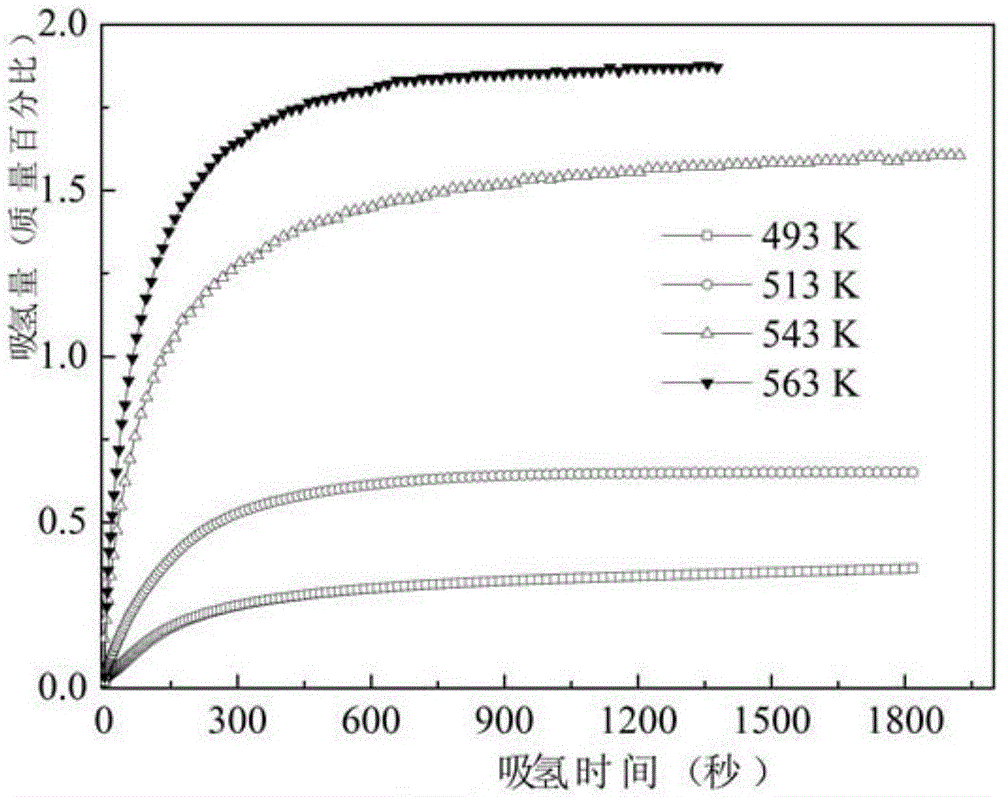
[0042] Put the blank C into a vacuum tube furnace for sintering, pass high-purity Ar as a protective gas, and use a fluid atmosphere to make the MgH 2 The hydrogen gas released by decomposition is discharged. The sintering temperature is 500° C., and the sintering time is 8 hours. Finally, the sample is taken out and crushed mechanically, and ball milled on a planetary ball mill to obtain a powder sample (a), the chemical composi...
Embodiment 2
[0044] According to the method in Example 1, replace Mn with Nb and Zr, obtain powder samples (b) and (c) respectively, the chemical composition is Mg respectively 2 Ni 0.7 Nb 0.3 , Mg 2 Ni 0.7 Zr 0.3 . The specific preparation method is different from Example 1 in that when preparing the powder sample (b), the rotational speed of the ball mill is set to 200 rpm, and the mixing time is set to 3 hours; the sintering temperature is 480°C, and the sintering time is 10 Hour. When preparing the powder sample (c), the speed of the ball mill was set at 300 rpm, and the mixing time was set at 4 hours; the sintering temperature was 580°C, and the sintering time was 5 hours.
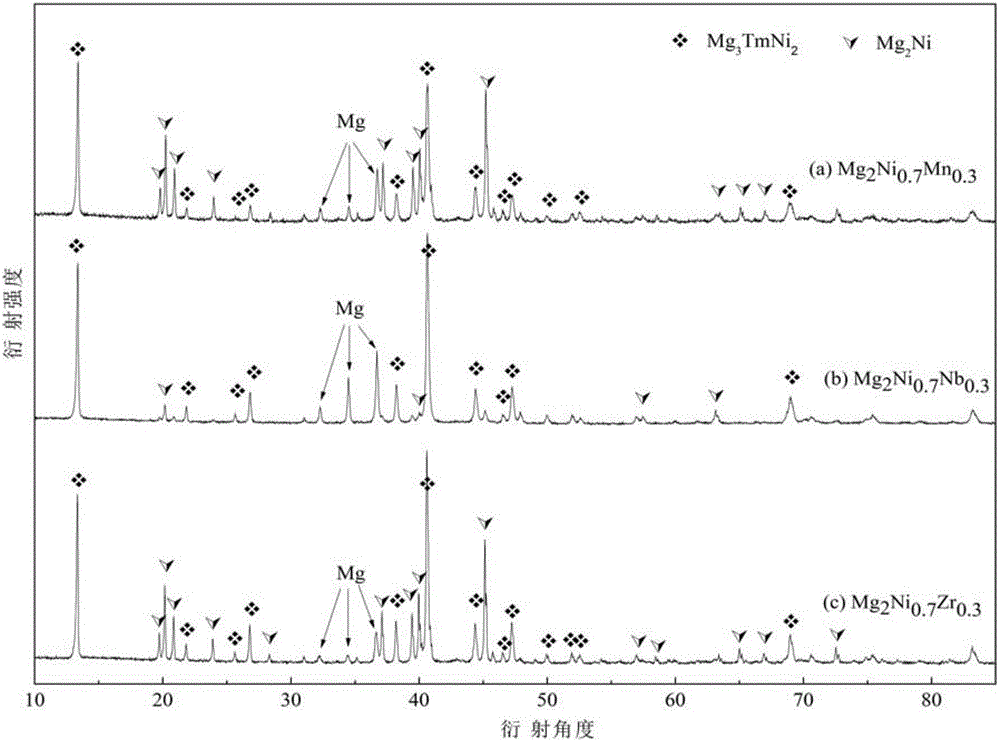
[0045] Sample (a) in Example 1 and samples (b) and (c) in Example 2 were subjected to XRD analysis test, and the XRD test analysis results were as follows figure 2 .
[0046] From figure 2 It can be seen that the intermetallic compound phase Mg with a face-centered cubic structure (FCC structure) is for...
Embodiment 3
[0048] Prepare Mg according to the method in embodiment 1 2 Ni 0.7 Nb 0.2 mn 0.1 with Mg 2 Ni, wherein the specific preparation method is different from Example 1 in that the preparation of Mg 2 Ni 0.7 Nb 0.2 mn 0.1 When, the MgH 2 , Ni, Nb and Mn are mixed according to the ratio of 20:7:2:1 (molar ratio) to prepare Mg 2 For Ni, put MgH 2 , Ni mixed according to the ratio of 2:1 (molar ratio).
[0049] The sample (a) in embodiment 1, the sample (b) in embodiment 2, (c) and the Mg prepared by this embodiment 2 Ni 0.7 Nb 0.2 mn 0.1 , Mg 2 Ni samples were fabricated into electrodes respectively. The specific production method is as follows:
[0050] (1) Weigh 2g powder sample, and mix 30wt.% high-purity nickel powder as a conductive agent, add an appropriate amount of binder PTFE, and fully grind it evenly in an agate bowl;
[0051] (2) Apply the uniformly mixed powder sample in (1) to the front and back sides of a 1cm×1cm foam nickel sheet;
[0052] (3) Wrap th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com