Method for measuring ambrisentan content through high performance liquid chromatography
A high-performance liquid chromatography and ambrisentan technology, which is applied in the field of high-performance liquid chromatography to determine the content of ambrisentan, achieves strict and effective control, and meets the needs of research and development and production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The amount of phosphate added in the phosphate buffer solution of phase A is 10mmol / L; the pH value of the phosphate is 2, and the mixing volume ratio of phase B and phase A is 40:60.
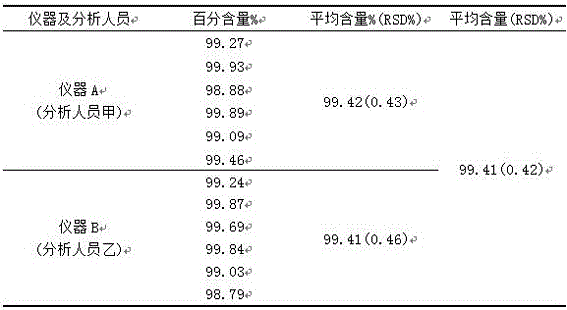
[0027] The specific operation steps of measuring ambrisentan content are:
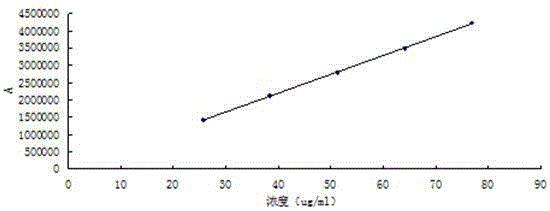
[0028] (1) Take 20mg ambrisentan tablet, weigh it accurately, put it in a measuring bottle, add diluent to dissolve and dilute to a solution containing 0.05mg ambrisentan per 1mL, as the test solution, the diluent is Mixture of water and acetonitrile, the mixing volume ratio of water and acetonitrile is 25:75;
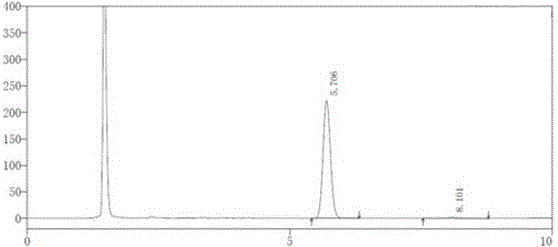
[0029] (2) Precisely measure 20 μl of the test solution, inject it into the liquid chromatograph, and record the chromatogram;
[0030] (3) Take 20mg ambrisentan tablet, weigh it accurately, place it in a measuring bottle, add diluent to dissolve and dilute to a solution containing 0.05mg ambrisentan per 1mL, as the reference solution, the diluent is water Mixed liquid with acetonitrile, the mixing volu...
Embodiment 2
[0034] The amount of phosphate added in the phosphate buffer solution of phase A is 60mmol / L; the pH value of phosphate is 4, and the mixing volume ratio of phase B and phase A is 60:40.
[0035] The specific operation steps of measuring ambrisentan content are:
[0036] (1) Take 150mg ambrisentan tablet, weigh it precisely, place it in a measuring bottle, add diluent to dissolve and dilute to a solution containing 0.05mg ambrisentan per 1mL, as the test solution, the diluent is Mixture of water and acetonitrile, the mixing volume ratio of water and acetonitrile is 25:75;
[0037] (2) Precisely measure 20 μl of the test solution, inject it into the liquid chromatograph, and record the chromatogram;
[0038] (3) Take 150mg ambrisentan tablet, weigh it precisely, place it in a measuring bottle, add diluent to dissolve and dilute to a solution containing 0.05mg ambrisentan per 1mL, as the reference solution, the diluent is water Mixed liquid with acetonitrile, the mixing volume...
Embodiment 3
[0042] The amount of phosphate added in the phosphate buffer solution of phase A is 35mmol / L; the pH value of phosphate is 3, and the mixing volume ratio of phase B and phase A is 45:55.
[0043] The specific operation steps of measuring ambrisentan content are:
[0044] (1) Take 85mg ambrisentan tablet, weigh it precisely, place it in a measuring bottle, add diluent to dissolve and dilute to a solution containing 0.05mg ambrisentan per 1mL, as the test solution, the diluent is Mixture of water and acetonitrile, the mixing volume ratio of water and acetonitrile is 25:75;
[0045] (2) Precisely measure 20 μl of the test solution, inject it into the liquid chromatograph, and record the chromatogram;
[0046] (3) Take 85mg ambrisentan tablet, weigh it accurately, place it in a measuring bottle, add diluent to dissolve and dilute to a solution containing 0.05mg ambrisentan per 1mL, as the reference solution, and the diluent is water Mixed liquid with acetonitrile, the mixing vol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com