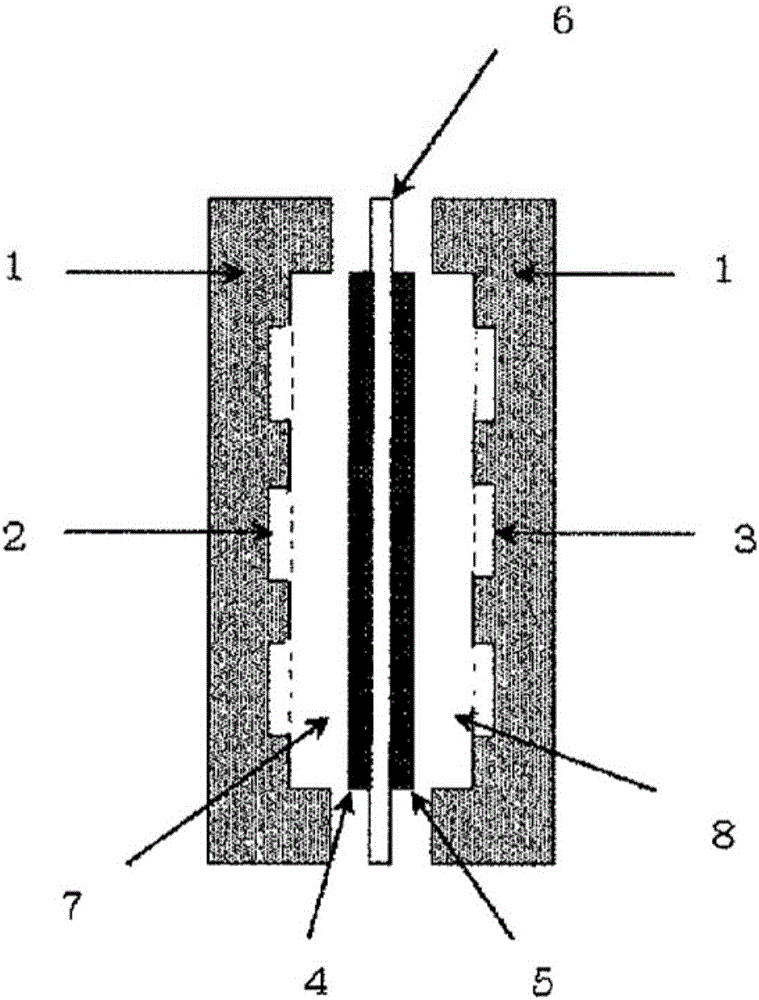
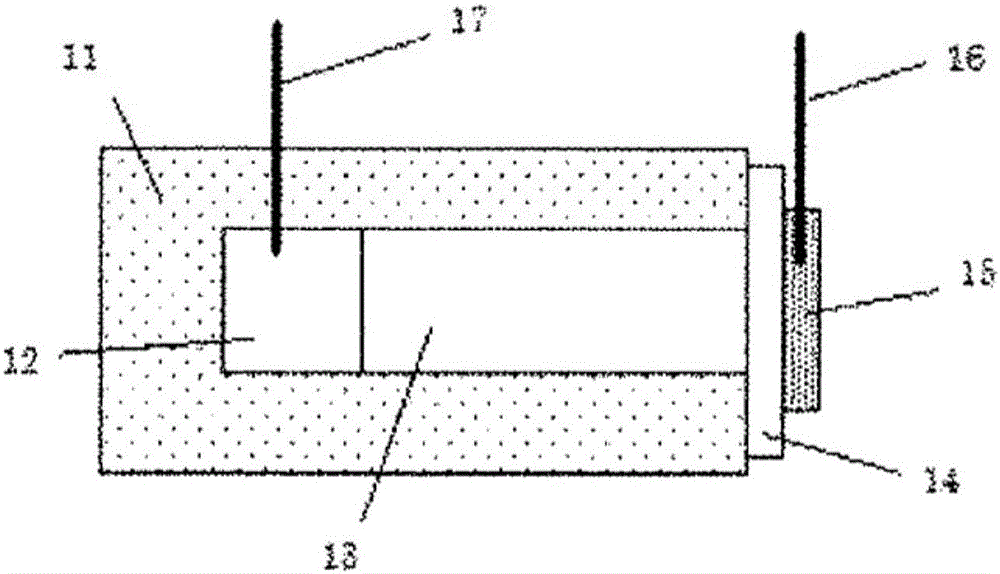
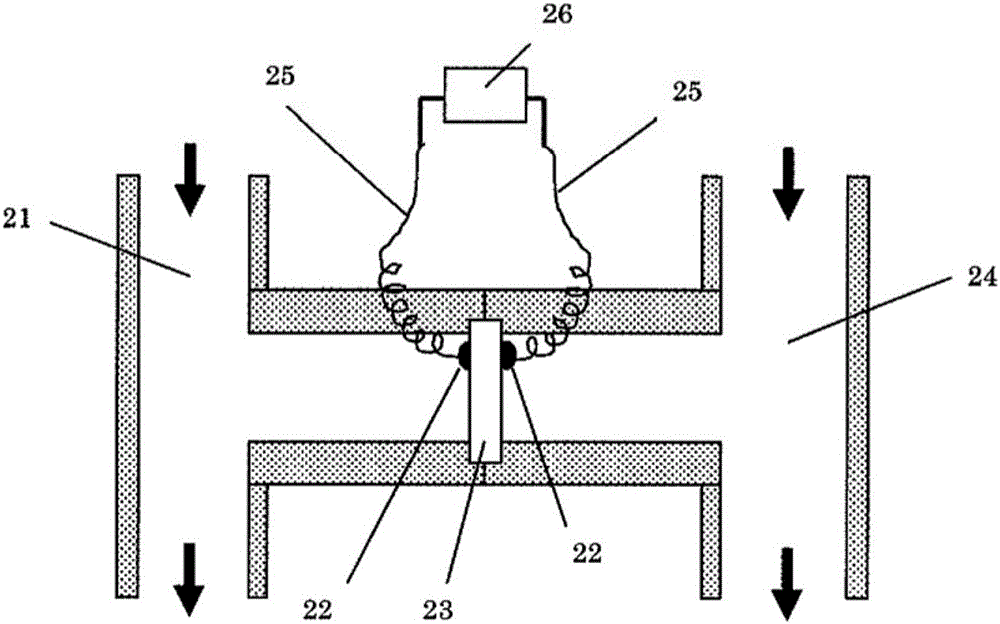
Anion conductor and layered metal hydroxide
A layered metal, hydroxide technology, applied in rare earth metal oxide/hydroxide, rare earth metal compound, yttrium oxide/yttrium hydroxide and other directions, can solve the problem of lack of heat resistance of anion exchange membrane, achieve physical Excellent properties, simple composition and easy control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1(︰ Y2(OH)5(NO3)-nH2O Synthetic example 1
[0099] By Y(NO of 0.44mol / l 3 ) 3 Aqueous solution 11.25ml, dropwise add 2.1mol / l NaOH and 1.44mol / l NaNO 3 3.75 ml of the mixed aqueous solution was stirred to obtain a white precipitate. The solution containing this white precipitate was put into an airtight container and left to stand at 25° C. for 30 hours. Thereafter, it was transferred to a centrifuge container, centrifuged (15,000 rpm, 30 minutes, 20° C.), and a white precipitate was collected. The white precipitate and water were put into a centrifugation container, and the operation (washing operation) of removing only water after centrifugation was repeated four times to remove remaining raw materials. Thereafter, 0.8 g of a white powdery product (compound 1) was obtained by vacuum drying at room temperature.
[0100] Analysis of the crystal structure by FT-IR (Fourier Transform Infrared Spectroscopy) and XRD confirmed that the crystal structure of compound 1 has a layered structure, and the composition is [Y 2 ...
Embodiment 2
[0101] Embodiment 2 (basic layered yttrium hydroxide: [Y 2 (OH) 5 (NO 3 )-nH 2 O] synthesis example 2)
[0102] A heat-resistant container was used for the airtight container, and the temperature when standing for 30 hours was 50, 75, 100, 125, 150°C, and the same operation as in Example 1 was carried out to obtain [Y 2 (OH) 5 (NO 3 )-nH 2 O] (Compounds 2-6). Table 1 shows the physical properties of the obtained compounds 2-6. When the temperature is raised, the half-width of the peak of the spectrum measured by XRD becomes smaller, and it can be seen that the particle size becomes larger.
[0103] [Table 1]
[0104]
Embodiment 3
[0105] Embodiment 3 ([Y 2 (OH) 5 (NO 3 ) x (CO 3 ) y -nH 2 O] synthesis example)
[0106] Y obtained in the same way as in Example 1 2 (OH) 5 (NO 3 ) powder 1.3g, impregnated in K at 25°C 2 CO 3 In aqueous solution for 7 days, the nitrate ions (NO 3 - ) to carbonate ion (CO 3 2- ) replacement. After the FT-IR analysis of the white powder (compound 7) after the cleaning operation, the result showed the presence of carbonate ions. - The peak of the asymmetric stretching vibration (1521cm -1 )Increase. From this, it can be confirmed that the substitution of carbonate ions proceeds.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com