Pharmaceutical composition of bezafibrate and application thereof to rheumatoid arthritis
A bezafibrate and rheumatoid technology, applied in the field of biomedicine, can solve the problems that have not been reported related to bezafibrate rheumatoid arthritis, etc., and achieve the goal of highlighting substantive features, improving effects, and making significant progress Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
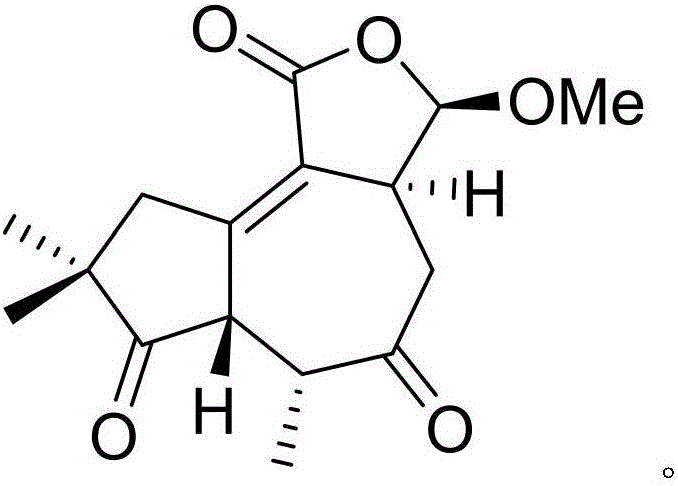
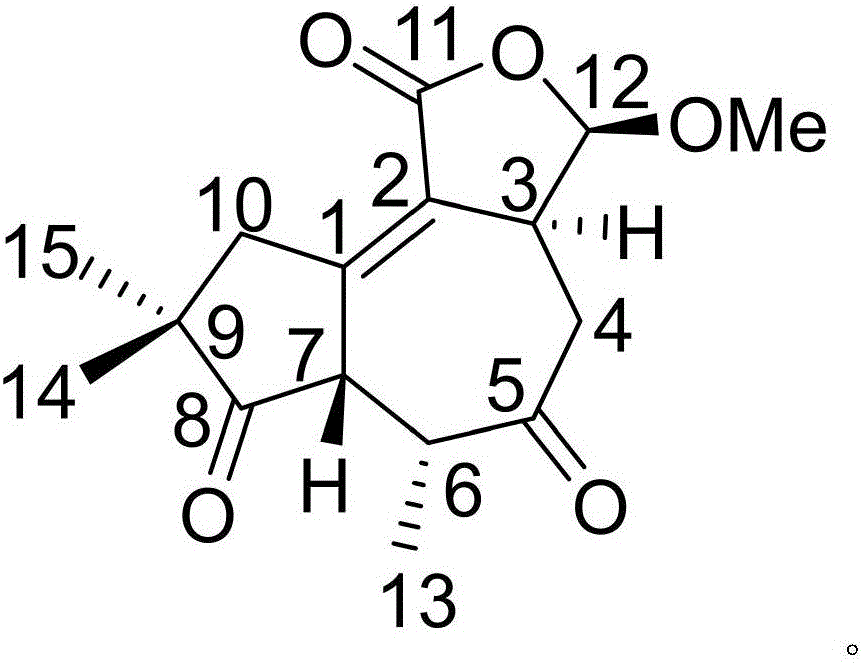
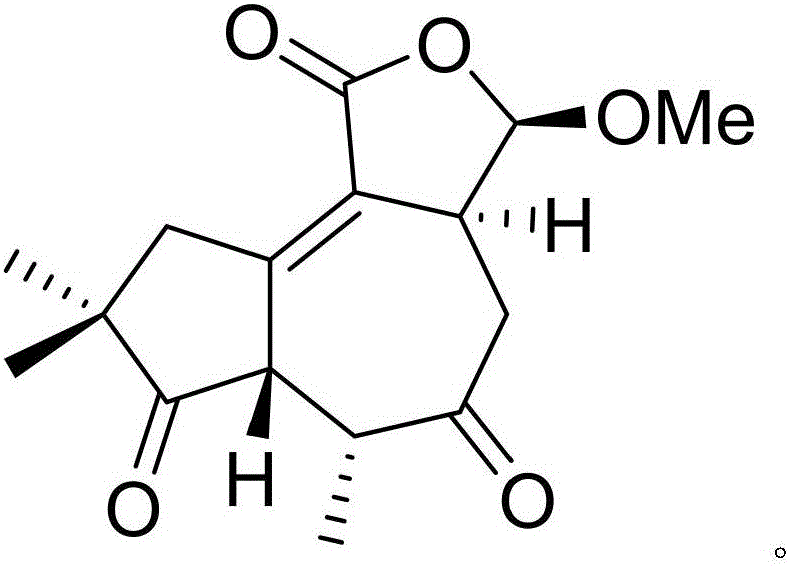
[0019] Example 1: Compound (I) Separation Preparation and Structure Confirmation
[0020] Separation method: (a) crush the dried flower buds (2kg) of cloves, extract with 80% ethanol under heat reflux (15L × 3 times), combine the extracts, concentrate until no alcohol smell (3L), and then use petroleum ether (3L× 3 times), ethyl acetate (3L × 3 times) and water-saturated n-butanol (3L × 3 times) were extracted to obtain petroleum ether extract, ethyl acetate extract and n-butanol extract respectively; (b) step (a) The ethyl acetate extract in (a) was removed with D101 type macroporous resin, first eluted with 20% ethanol for 12 column volumes, then with 80% ethanol for 15 column volumes, collected 80% eluate, and subtracted Concentrate under pressure to obtain 80% ethanol elution concentrate; (c) in step (b), 80% ethanol elution concentrate is separated with normal phase silica gel, and the volume ratio is 80:1 (10 column volumes), 40:1 successively (8 column volumes), 20:1 (...
Embodiment 2
[0024] Embodiment 2: pharmacological action
[0025] 1. Materials and methods
[0026] 1.1 Animals
[0027] Male Wistar rats (provided by the Animal Experiment Center of Tongji Medical College, Huazhong University of Science and Technology) with a body weight of about 120 g were selected.
[0028] 1.2 Reagents and samples
[0029] Bezafibrate was purchased from China National Institute for the Control of Pharmaceutical and Biological Products. Compound (I) is self-made, and the preparation method is shown in Example 1. Type Ⅱ collagen (Sigma, USA), incomplete Freund's adjuvant (Sigma), and RPMI-1640 (GIBCO).
[0030] 1.3 Instruments
[0031] American Nurie CO 2 Incubator (NU4750 type), Japanese Olympian inverted fluorescence microscope (CKX41 type), American Beckman Coulter flow cytometer (EPICSXL type), Japanese transmission electron microscope (Hitachi H-7500 type).
[0032] 1.4 Rat model preparation and cell grouping
[0033] Preparation of CIA Rat Model Dissolve ty...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com