A liposome preparation for nasal administration of teriparatide and its preparation method
A technology of liposome preparation and teriparatide, which is applied in the field of nasal administration liposome and its preparation, can solve the problems of reduced activity of polypeptide drugs, difficulty in realizing industrialization, and low encapsulation efficiency, and achieves low toxicity, Effect of prolonging residence time and improving bioadhesion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
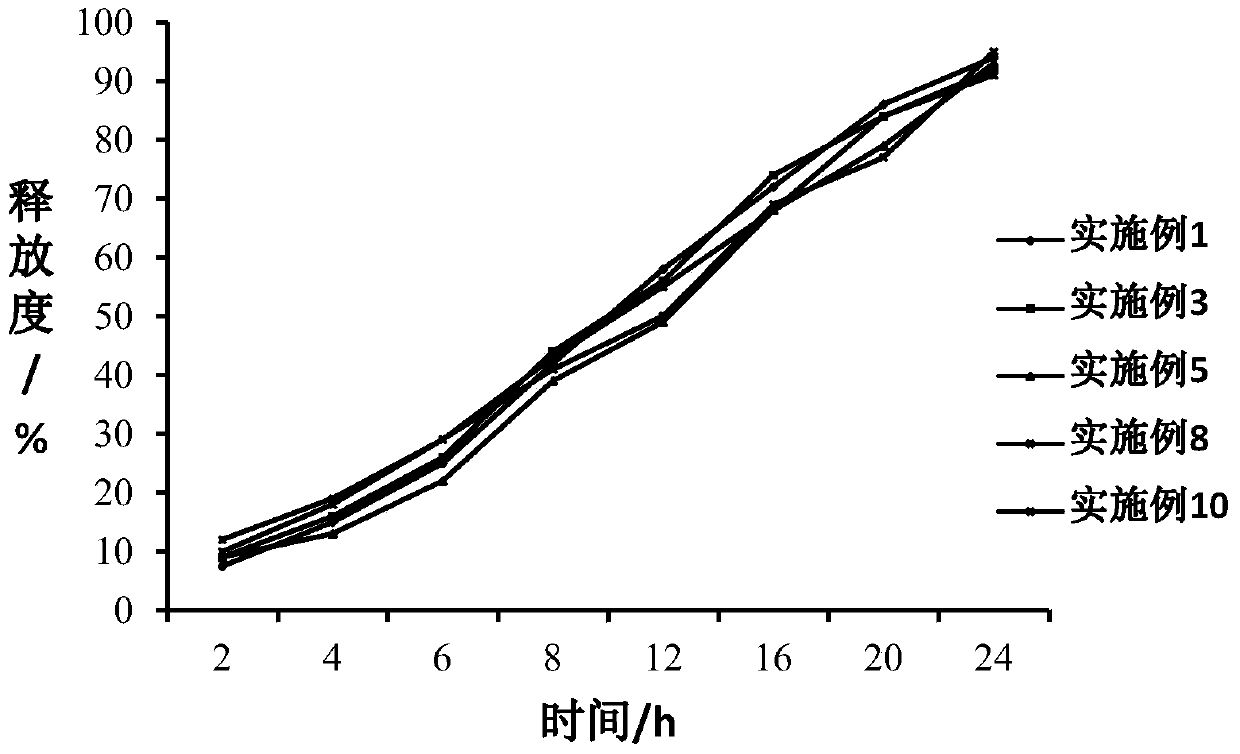
Examples
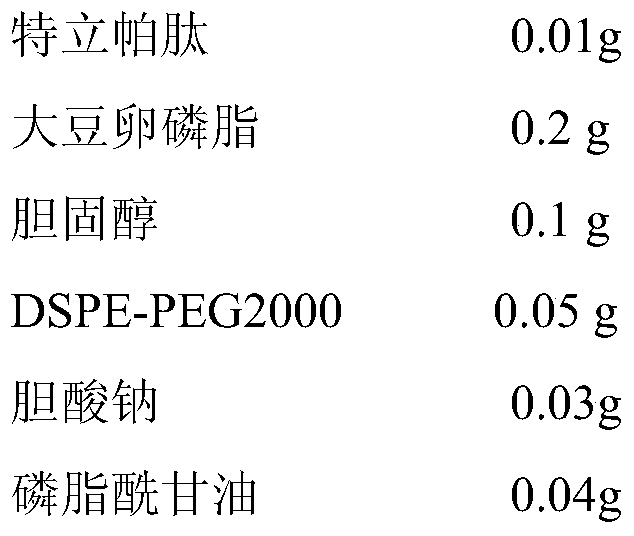
Embodiment 1
[0030]
[0031] a) Teriparatide, soybean lecithin, cholesterol, DSPE-PEG2000 and sodium cholate were dissolved in supercritical CO 2 / ethanol solvent, and solubilize a certain amount of distilled water to form a supercritical microemulsion;
[0032] b) pre-expand the supercritical microemulsion under the conditions of set pressure (16MPa) and temperature (335K); quickly spray the phosphate buffer solution in the supercritical collection tank through a nozzle at a certain flow rate (3L / min) In the medium (pH is 5.5), through dispersion and precipitation, liposome suspension is formed;
[0033] c) CO 2 Continue to pass through the supercritical collection kettle to dissolve and remove the residual ethanol in the liposome suspension, and empty it after measuring with the rotameter to collect the nanoliposome suspension in the supercritical collection kettle, which is Teripa Peptide flexible nanoliposomes;
[0034] d) adding phosphatidylglycerol to the teriparatide flexible ...
Embodiment 2
[0037]
[0038] a) Teriparatide, egg yolk lecithin, cholesterol, DSPE-PEG2000 and sodium deoxycholate were dissolved in supercritical CO 2 / ethanol solvent, and solubilize a certain amount of distilled water to form a supercritical microemulsion;
[0039] b) The supercritical microemulsion is pre-expanded under the conditions of set pressure (20MPa) and temperature (328K); with a certain flow rate (2.5L / min), it is quickly sprayed into the phosphate buffer in the supercritical collection tank through the nozzle In the solution medium (pH is 6.5), after dispersion and precipitation, liposome suspension is formed;
[0040] c) CO 2 Continue to pass through the supercritical collection kettle to dissolve and remove the residual ethanol in the liposome suspension, and empty it after measuring with the rotameter to collect the nanoliposome suspension in the supercritical collection kettle, which is Teripa Peptide flexible nanoliposomes;
[0041] d) adding phosphatidic acid to ...
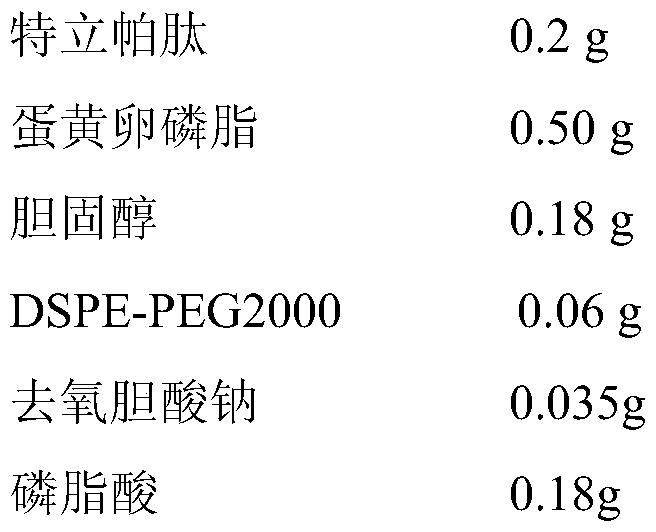
Embodiment 3
[0044]
[0045] a) Teriparatide, egg yolk lecithin, cholesterol, DSPE-PEG2000 and sodium deoxycholate were dissolved in supercritical CO 2 / ethanol solvent, and solubilize a certain amount of distilled water to form a supercritical microemulsion;
[0046] b) The supercritical microemulsion is pre-expanded under the conditions of set pressure (25MPa) and temperature (338K); with a certain flow rate (3.5L / min), it is quickly sprayed into the phosphate buffer in the supercritical collection tank through the nozzle In solution medium (pH is 7.0), after dispersion and precipitation, liposome suspension is formed;
[0047] c) CO 2 Continue to pass through the supercritical collection kettle to dissolve and remove the residual ethanol in the liposome suspension, and empty it after measuring with the rotameter to collect the nanoliposome suspension in the supercritical collection kettle, which is Teripa Peptide flexible nanoliposomes;
[0048] d) adding stearylamine to the teripar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com