Loratadine nasal cavity in situ gel and preparation method thereof
A technology of loratadine and in-situ gel, which is applied in pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc., can solve gastrointestinal adverse reactions, liver function, and effective utilization. It can improve bioavailability, reduce toxicity, and improve clearance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
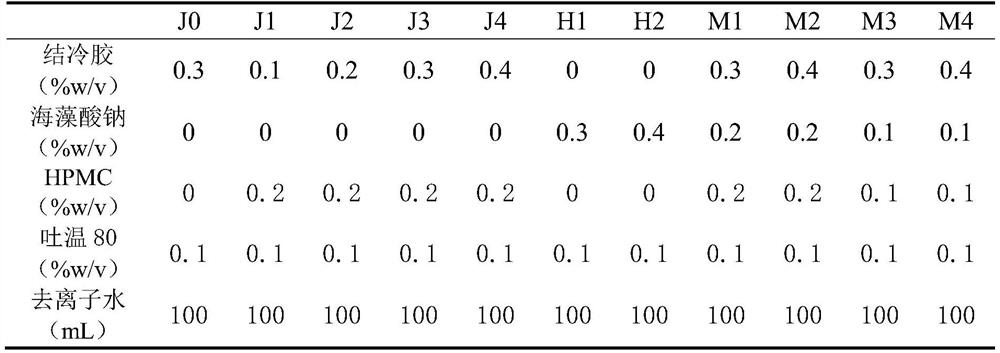
[0024] The present invention also provides a preparation method of the loratadine nasal in situ gel, the steps are as follows: according to the formula, gellan gum is placed in a reaction vessel, heated and stirred until completely dissolved, then taken out and allowed to cool, Add sodium alginate, HPMC, Tween 80, and deionized water, and stir until completely dissolved.
[0025] In one embodiment, the temperature of the heating and stirring is 87°C.
Embodiment 1
[0028] The instrument used in the present invention is as follows:
[0029] CL-1A magnetic stirrer (Gongyi Yingyu high-tech instrument factory); DF-Ⅱ digital display heat-collecting magnetic stirrer (Jintan Jerry Electric Co., Ltd.); 85-2 constant temperature magnetic stirrer ( Shanghai Sile Instrument Factory); FA2004B electronic balance (Shanghai Yueping Scientific Instrument (Suzhou) Manufacturing Co., Ltd.); PS-30AL ultrasonic cleaning machine (Shenzhen Shenhuatai Ultrasonic Cleaning Equipment Co., Ltd.); NDJ-1B rotary Viscometer (Shanghai Changji Geological Instrument Co., Ltd.); DHG-9035A blast drying oven (Shanghai Aozhen Instrument Manufacturing Co., Ltd.); s53 UV-Vis spectrophotometer (Shanghai Prism Technology Co., Ltd.); RYJ-12B drug Transdermal diffusion tester; smart phone (Huawei Honor V10 low configuration version).
[0030] The materials used in the present invention are as follows:
[0031] Gellan gum (OPAL Biotech, batch number 18042510); hypromellose (Shan...
Embodiment 2
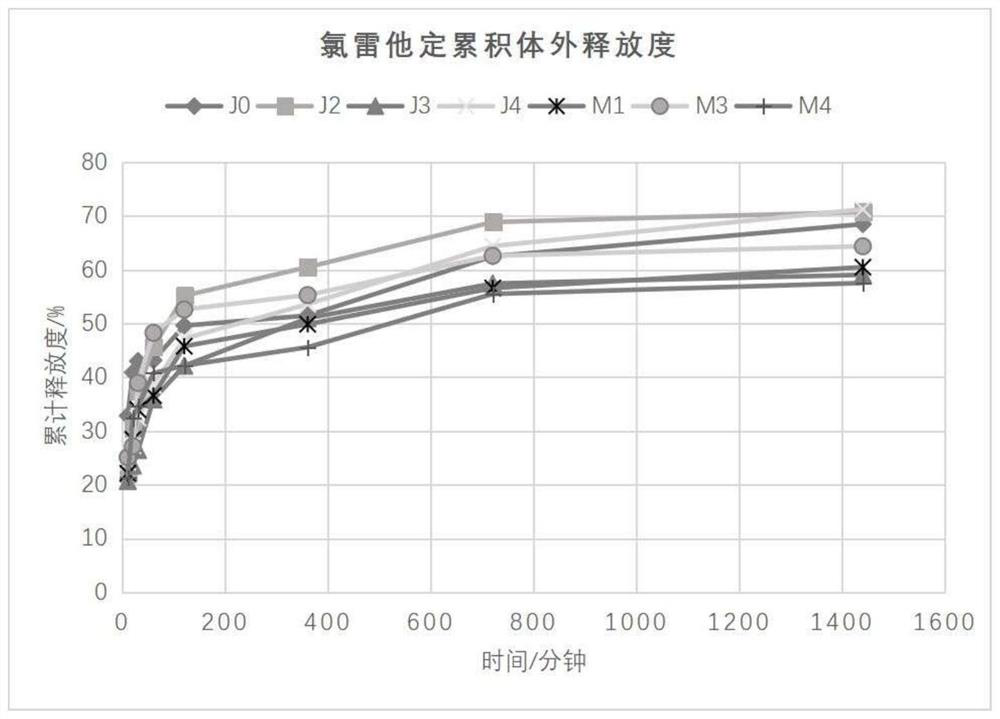
[0051] Preparation of Loratadine Nasal In Situ Gel: Weigh 0.7624g of Loratadine crude drug, transfer it to a 10mL volumetric flask, add 98% ethanol to the scale line to make up the volume, and ultrasonically dissolve it for later use. Prepare the blank gel according to the prescription in Table 1 and the method described in 2.3.2, take out 10g and put it in a 15mL centrifuge tube, add 1mL of loratadine-ethanol solution into the taken out gel, stir it evenly with a glass rod, and use it as the sample to be tested .
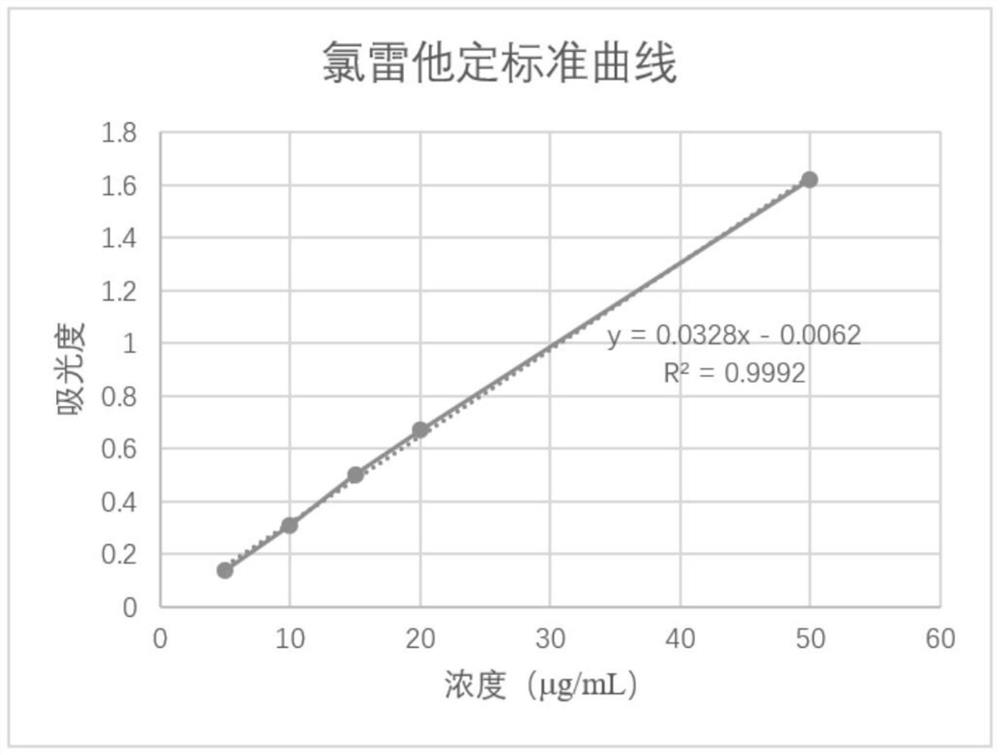
[0052] 1. Determination of loratadine content: It is known that with 50% ethanol aqueous solution as solvent, loratadine has ultraviolet absorption at 247nm, and other substances in the preparation do not absorb under this wavelength, so ultraviolet-visible spectrophotometry can be used Determination of loratadine content.
[0053] Drawing of the standard curve of loratadine solution: Weigh 125.3mg of loratadine, transfer to a 500mL volumetric flask, dilute to the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com