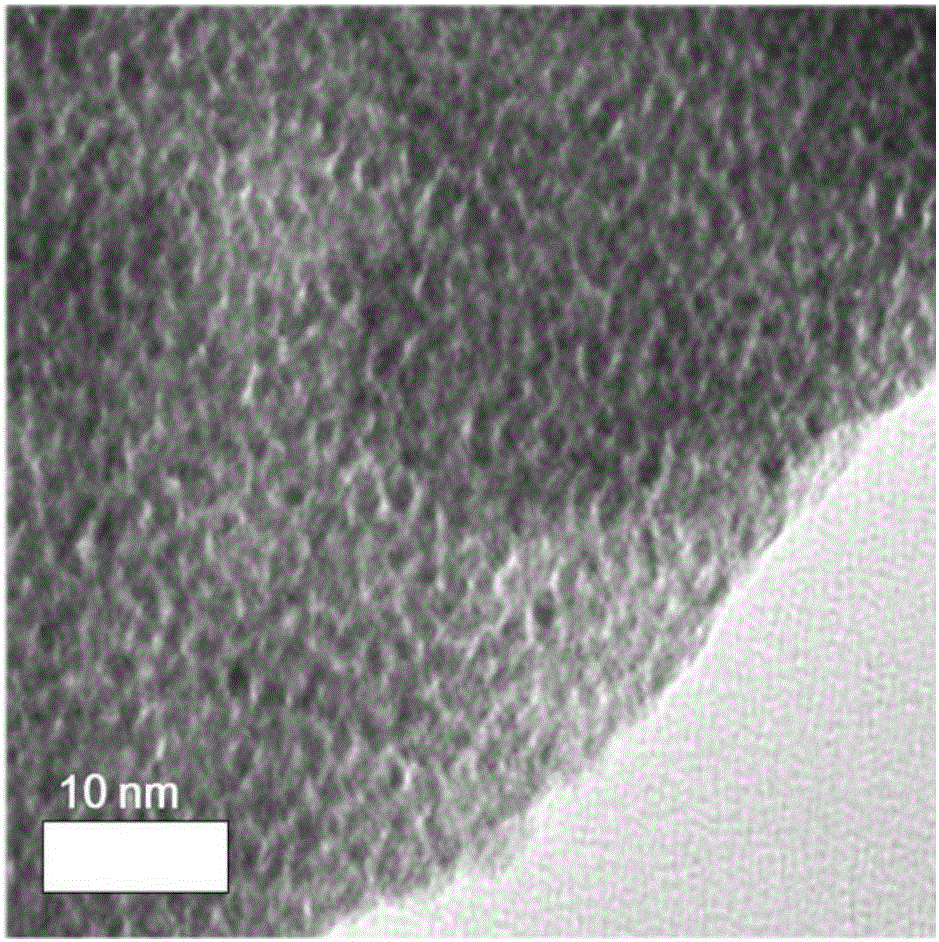
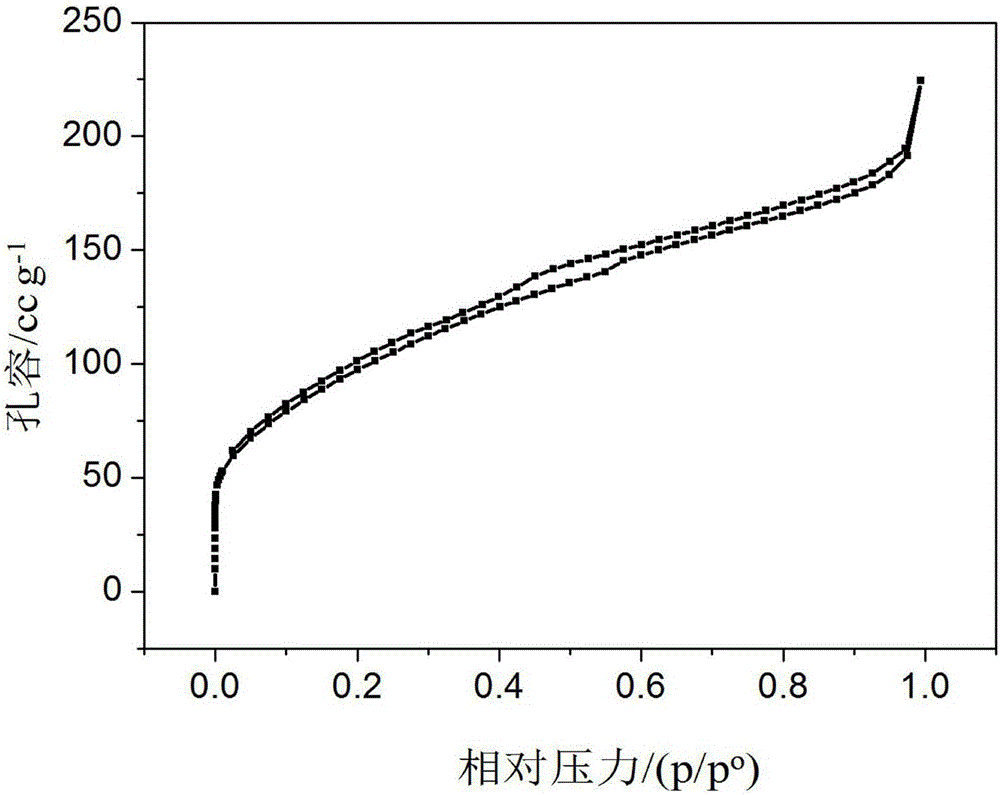
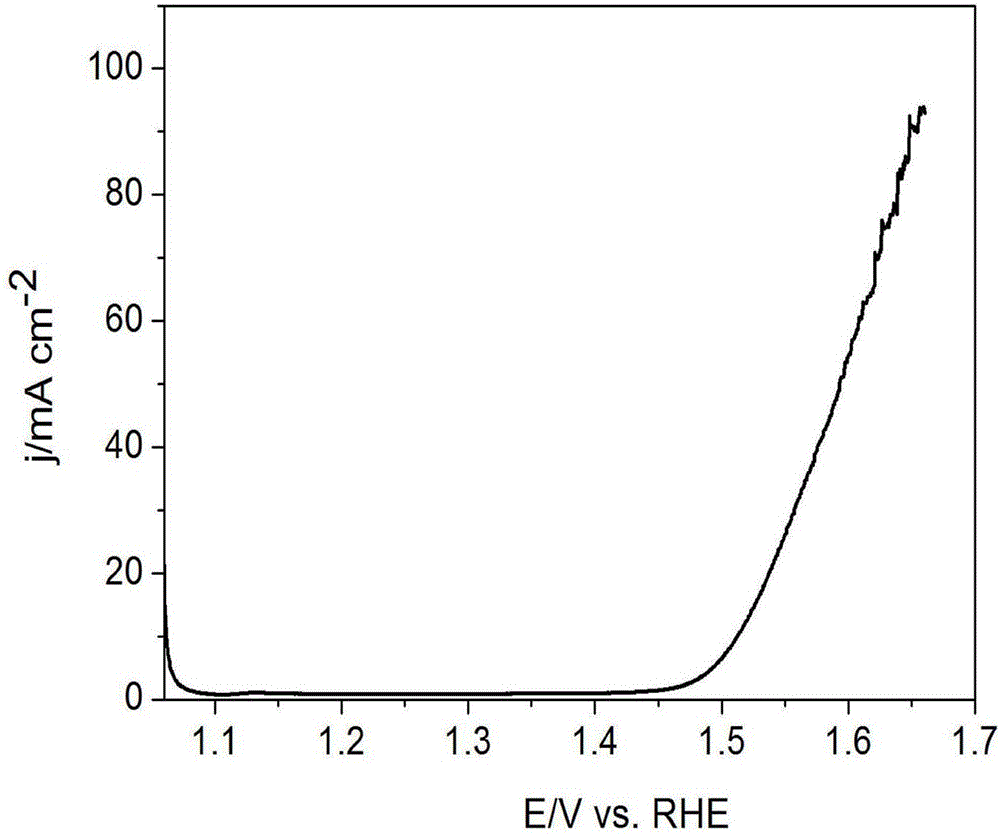
Porous IrO2 oxygen evolution catalyst with super-high specific surface area and preparation method of porous IrO2 oxygen evolution catalyst
An ultra-high specific surface area and catalyst technology, applied in the field of electrochemistry, can solve the problems of increasing the difficulty of controlling the structure and pore structure, accelerating proton transfer, reducing the active area of catalysts, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037]The present invention firstly provides a porous ultra-high specific surface area IrO 2 A method for preparing an oxygen evolution catalyst, the method comprising:
[0038] Step 1: H 2 IrCl 6 ·6H 2 The O precursor is added to deionized water to obtain a first solution;
[0039] Step 2: Add NH to the first solution obtained in Step 1 3 ·H 2 O reacts to obtain the second solution;
[0040] Step 3: Add NaNO to the second solution obtained in Step 2 3 Reaction, obtain the 3rd solution;
[0041] Step 4: Evaporate the solvent in the third solution obtained in step 3 to dryness, and burn the product obtained in the air to obtain porous ultra-high specific surface area IrO 2 Oxygen evolution catalyst.
[0042] According to the present invention, H 2 IrCl 6 ·6H 2 The O precursor is added to deionized water to obtain a first solution, and the concentration of the first solution is preferably 0.005-0.02mol L -1 .
[0043] According to the present invention, NH is added...
Embodiment 1
[0060] 1) put H 2 IrCl 6 ·6H 2 The O precursor was added to deionized water to obtain the first solution with a concentration of 0.01 mol L -1 .
[0061] 2) Add NH to the first solution in 1) 3 ·H 2 O, H 2 IrCl 6 ·6H 2 O and NH 3 ·H 2 The molar ratio of O was 1:100, and ultrasonic reaction was performed for 3 h to obtain the second solution.
[0062] 3) adding NaNO to the second solution in 2) 3 , H 2 IrCl 6 ·6H 2 O and NaNO 3 The mass ratio of the solution was 1:20, and the ultrasonic reaction was performed for 1 h to obtain the third solution.
[0063] 4) The third solution in 3) was reacted in a water bath at 80° C. until the solvent was evaporated to dryness to obtain the first product.
[0064] 5) Burning the first product described in 4) in an air atmosphere for 0.5h, the burning temperature is 450°C,
[0065] Cool to room temperature, wash, filter with suction, and dry overnight under vacuum at 60°C to obtain the IrO 2 catalyst.
[0066] Embodiment 1 ...
Embodiment 2
[0069] 1) put H 2 IrCl 6 ·6H 2 The O precursor was added to deionized water to obtain the first solution with a concentration of 0.005 mol L -1 .
[0070] 2) Add NH to the first solution in 1) 3 ·H 2 O, H 2 IrCl 6 ·6H 2 O and NH 3 ·H 2 The molar ratio of O was 2:20, and the ultrasonic reaction was performed for 4 hours to obtain the second solution.
[0071] 3) adding NaNO to the second solution in 2) 3, H 2 IrCl 6 ·6H 2 O and NaNO 3 The mass ratio of the solution was 1:20, and the ultrasonic reaction was performed for 2 hours to obtain the third solution.
[0072] 4) The third solution in 3) was reacted in a water bath at 80° C. until the solvent was evaporated to dryness to obtain the first product.
[0073] 5) Burning the first product described in 4) in an air atmosphere for 0.5h, the burning temperature is 450°C,
[0074] Cool to room temperature, wash, filter with suction, and dry overnight under vacuum at 60°C to obtain the IrO 2 catalyst.
[0075] Wi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com