Preparation process of bupivacaine hydrochloride
A kind of technology of bupivacaine hydrochloride and preparation process, which is applied in the production field of bupivacaine hydrochloride, can solve the problems of high production cost, large amount of DMF usage, difficulty in recycling, etc., and achieve the reduction of three wastes, production cost, and Effects of Environmental Pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Add 500g of toluene to a 1L four-necked reaction flask, then add 100g of starting materials, then add 1.01 to 1.05 times the equivalent of anhydrous potassium carbonate, then add 2% to 6% of tetrabutylammonium bromide, and then add 1.1 to 1.3 times the equivalent of n-bromobutane, slowly heat up to 80-85°C, keep the temperature for 5 hours, cool down to room temperature, filter, transfer the filtrate to a 1L four-necked flask, and pass hydrogen chloride gas into the filtrate until no longer Filter until absorbed, wash the filter cake with toluene, and dry to obtain 132.84-141.70 g of bupivacaine hydrochloride, with an HPLC content of >99% and a yield of 90%-96%.
Embodiment 2
[0027] Add 500g of toluene to a 1L four-necked reaction flask, then add 100g of starting materials, then add 1.01 to 1.05 times the equivalent of anhydrous sodium carbonate, then add 2% to 6% tetrabutylammonium bisulfate, and then add 1.1 to 1.3 times the equivalent of n-bromobutane, slowly heat up to 80-85°C, keep the temperature for 10 hours, cool down to room temperature, filter, transfer the filtrate to a 1L four-necked flask, slowly heat up to 80-85°C, add 160g 10% hydrochloric acid solution, stir to form salt, keep warm at 80-85°C and let it stand for stratification. The water layer is divided into a 500ml four-neck flask while it is hot, cool down to 5-10°C to crystallize, filter, and dry the filter cake to obtain bupika hydrochloride. Because 120.82~126.78g, HPLC content>99%, yield 81%~85%.
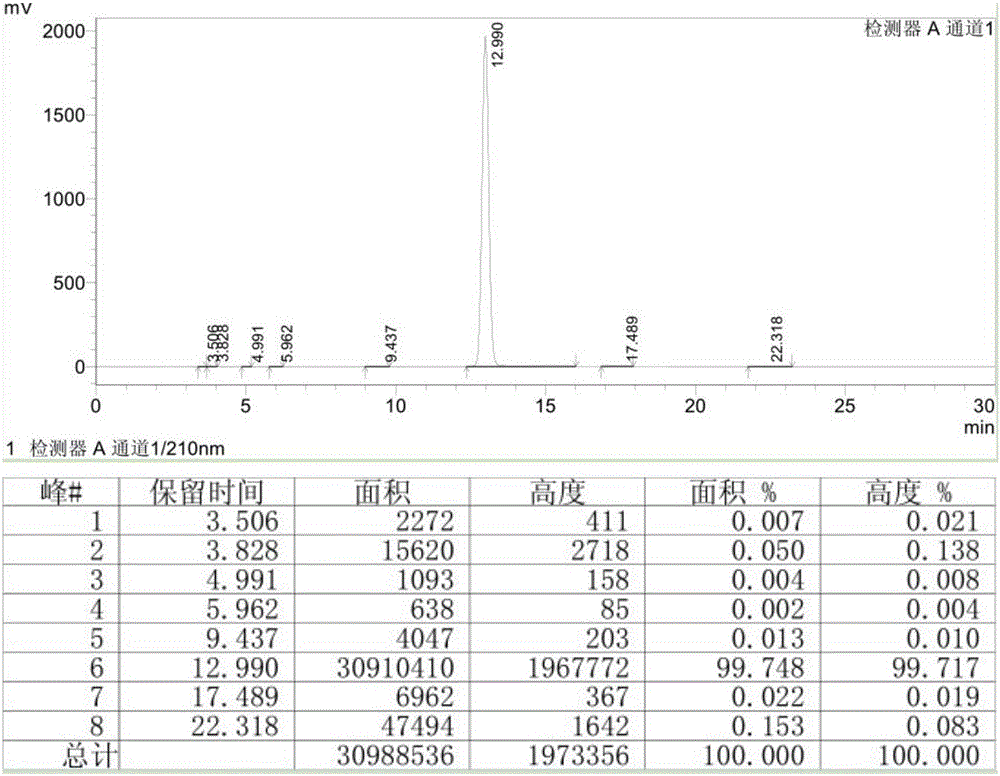
[0028] From figure 1 It can be concluded that the purity of bupivacaine hydrochloride directly obtained in Example 1 can reach more than 99.7%, and the purity is relatively high....
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com