New ibrutinib crystal form and preparation method thereof
A technology of ibrutinib and crystal form, which is applied in the field of medicinal chemistry and can solve problems such as unsuitable preparation development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0055] The present invention also provides a preparation method of the crystal III, comprising the steps of:
[0056] (i) dissolving crude ibrutinib I in an organic solvent, with a weight-to-volume ratio of 1:1-1:30 g / ml;
[0057] (ii) crystallization at -10°C to 90°C to obtain the crystal form III;
[0058] Wherein, the crude ibrutinib I is selected from the following group: ibrutinib crystal form C, ibrutinib amorphous substance, ibrutinib crystal form A, or a combination thereof.
[0059] In another preferred example, in the step (i), the organic solvent is selected from the group consisting of alcohol solvents, ester solvents, ketone solvents, alkanes, or combinations thereof, preferably alcohol solvents A mixed solvent with an alkane, a mixed solvent with an ester solvent and an alkane, a mixed solvent with a ketone solvent and an alkane, or a combination thereof.
[0060] As used herein, the number of carbon atoms of each of the alcohol solvents, ester solvents, ketone...
Embodiment 1
[0093] Example 1 Preparation of ibrutinib crystal form III
[0094] Add amorphous ibrutinib (5.0 g) into 50 ml of n-propanol, and heat to reflux to dissolve. Continue stirring for 30 minutes after dissolving. Stop heating, cool down at a speed of about 1 °C / min, cool to 50 °C, solids precipitate out, keep stirring for 2 hours, filter, the filtrate is rinsed with ethyl acetate, and the filter cake is vacuum-dried at 50 °C to obtain 3.2 g of product.
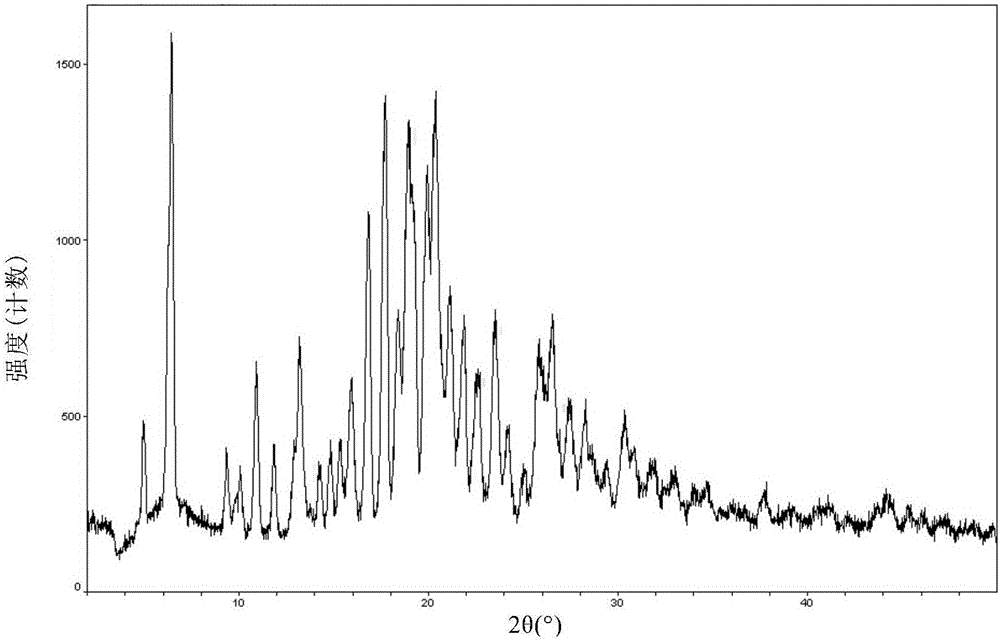
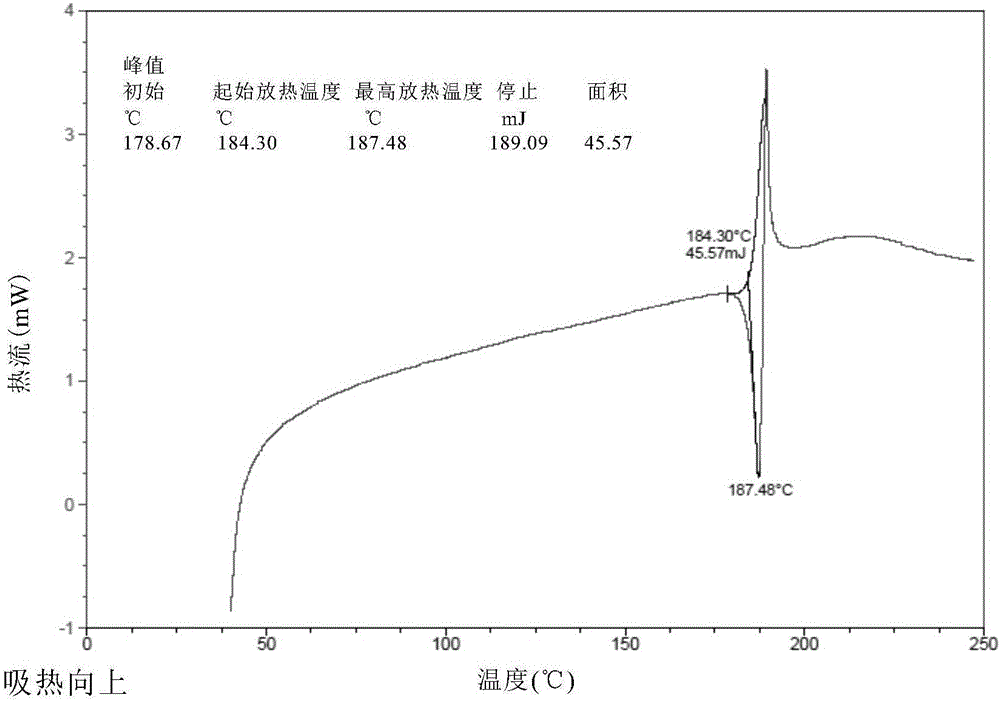
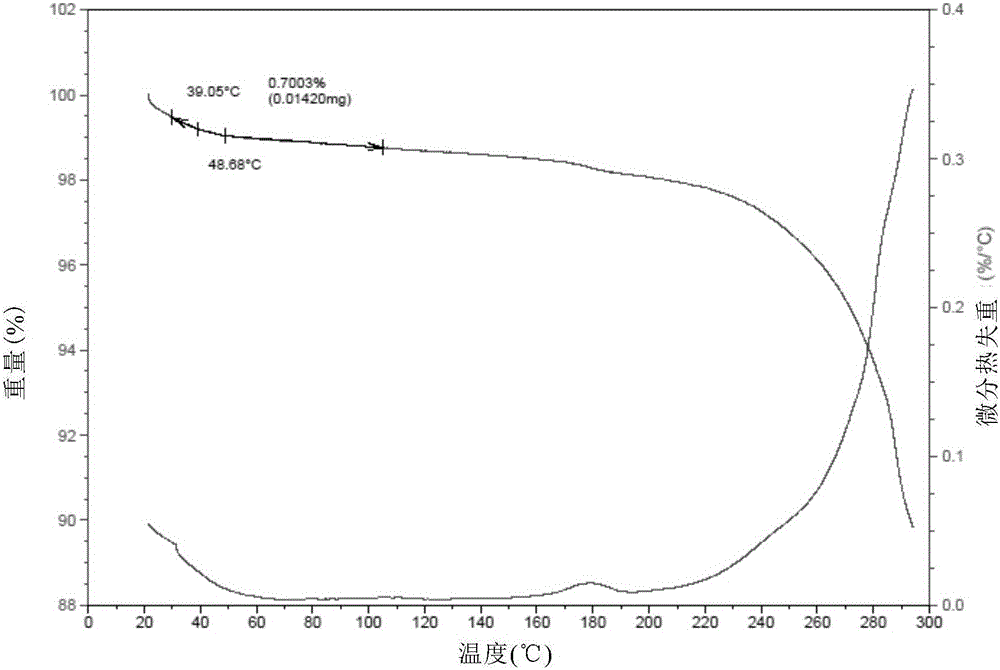
[0095] Result: The powder X-ray diffraction pattern of the obtained solid is as follows figure 1 Characterized by the differential thermal scanning spectrum as figure 2 As shown, thermogravimetric analysis such as image 3 As shown, the infrared Fourier transform spectrum is shown as Figure 4 shown.
Embodiment 2
[0096] Example 2 Preparation of ibrutinib crystal form III
[0097] Ibrutinib Form C (5.0 g) was added to 75 ml of isopropanol, stirred overnight at room temperature, the resulting suspension was filtered, and vacuum-dried at 50° C. to constant weight to obtain 3.9 g of a white solid.
[0098] Result: the powder X-ray diffraction pattern of the obtained solid is the same as the crystal form obtained in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com