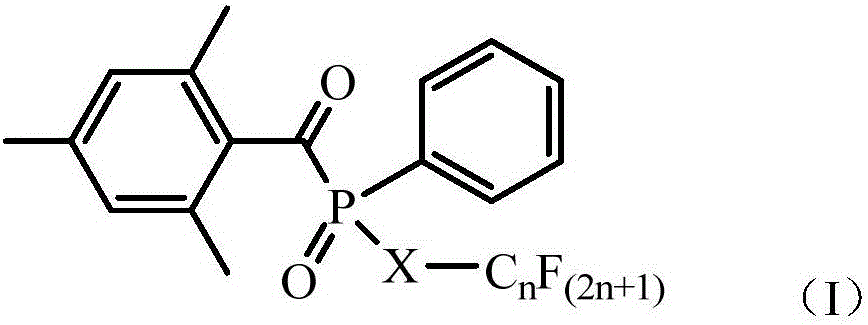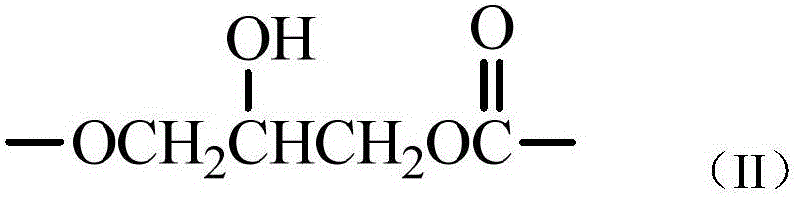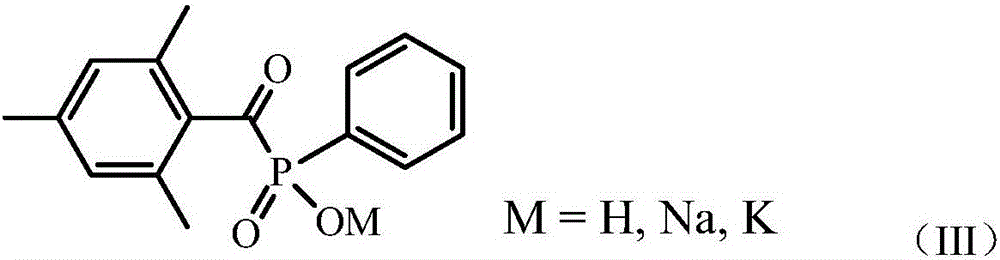Phosphonic acid ester photoinitiator containing fluorine-carbon chains and preparation method of phosphonic acid ester photoinitiator
A technology of photoinitiator and fluorocarbon chain, which is applied in the field of photosensitive materials and photocurable materials, can solve the problems of low absorption capacity, surface oxygen inhibition, and low free radical efficiency, and achieve low surface tension and overcome surface oxygen inhibition. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Add 0.095mol of sodium 2,4,6-trimethylbenzoylphenylphosphonate and 0.10mol of perfluorooctylethyl iodide in 200ml of toluene, stir rapidly, heat at 60°C for 20 hours, take a small amount of precipitated insoluble matter, and wash with acetone Decant, dry with hot air, carry out infrared spectrum test, confirm that 2900cm in the infrared absorption spectrum -1 Nearby and 1658cm -1 The characteristic absorption peak representing 2,4,6-trimethylbenzoylphenylphosphonate disappeared completely, indicating that the reaction was complete. Finish the reaction, filter, and distill the filtrate under reduced pressure to remove the solvent to obtain 72.5 g of liquid product. Mass spectrometry test proved to contain the molecular ion peak 734 corresponding to the target product.
Embodiment 2
[0027] 0.095mol of potassium 2,4,6-tritoluoylphenylphosphonate and 0.10mol of perfluorobutylethyl bromide in 200ml of butyl acetate, stirred rapidly, heated at 80°C for 10 hours, and took a small amount of precipitated insoluble matter, Wash with acetone, dry with hot air, carry out infrared spectrum test, confirm that the characteristic structure of benzoyl phosphonate disappears, and the reaction is complete. Finish the reaction, filter, and distill the filtrate under reduced pressure to remove the solvent to obtain 52.8 g of liquid product. Mass spectrometry test proved to contain the molecular ion peak 534 corresponding to the target product.
Embodiment 3
[0029] 0.095mol of sodium 2,4,6-trimethylbenzoylphenylphosphonate and 0.1mol of perfluorooctadecylethyl iodide in 200ml of cyclohexanone, stirred rapidly, heated at 80°C for 16 hours, a small amount of precipitate was insoluble The object was cleaned with acetone, dried with hot air, and tested by infrared spectroscopy, which confirmed that the characteristic structure of benzoyl phosphonate disappeared and the reaction was complete. Finish the reaction, filter, and distill the filtrate under reduced pressure to remove the solvent to obtain 122.9 g of liquid product. Mass spectrometry test proved to contain molecular ion peak 1234 corresponding to the target product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com