Synthesizing method of reticular cross-linked zinc polyacrylic acid and using method of synthesized product
A polyacrylic acid zinc, network cross-linking technology, applied in the direction of alkaline storage battery, nickel storage battery, etc., can solve the problems such as no explicit indication, achieve energy saving and emission reduction contribution, improve energy use efficiency, and increase power output
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] For a synthetic method of network cross-linked zinc polyacrylate, the synthesis is carried out according to the following four steps and corresponding conditions.
[0081] The first step, prepare materials:
[0082] Select the water-phase synthesis method, and prepare the five raw materials required for the water-phase synthesis, including: zinc oxide, acrylic acid, N N'-methylenebisacrylamide, potassium persulfate and distilled water. Except for distilled water, the other four All chemicals are chemically pure.
[0083] The second step, identification and initial ingredients:
[0084] Zinc oxide powder in barrels is packed in ziplock bags, marked as component A. Acrylic acid was formulated as a 50 wt.% aqueous solution of acrylic acid, which was identified as component B. Potassium persulfate is formulated into a 2wt.% aqueous solution, which is identified as D component. N N'-methylenebisacrylamide was formulated into a 2wt.% aqueous solution and identified as com...
Embodiment 2
[0138] Step, operation and characterization method are identical with implementation 1; The difference between condition and characterization result and embodiment 1 is:
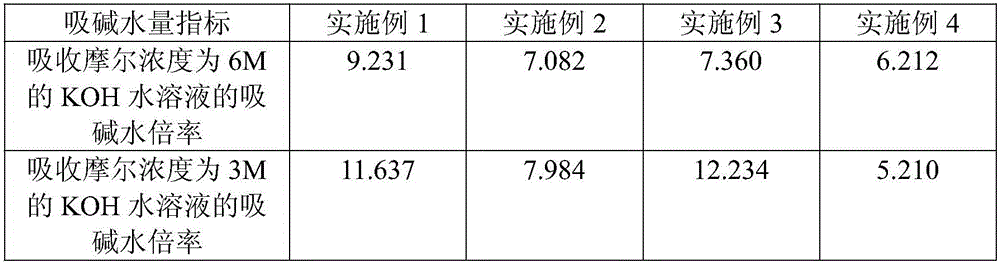
[0139] Ⅰ. Change the formula in the "third step" and "mixing" of Implementation 1 to: Add 5.078 kg of B component to 1 kg of A component, add 7.641 kg of C component, add 1.172 kg of D component and add 1.172 kg of E component Kilogram.
[0140] Ⅱ. Change the "replacement ratio" in the "replacement" in the "fifth step" of implementation 1 to: the dry product powder of zinc polyacrylic acid reticular cross-linking accounts for 5wt.% of the original zinc oxide feed. The particle size selection of dry powder is changed to 350 mesh ± 50 mesh.
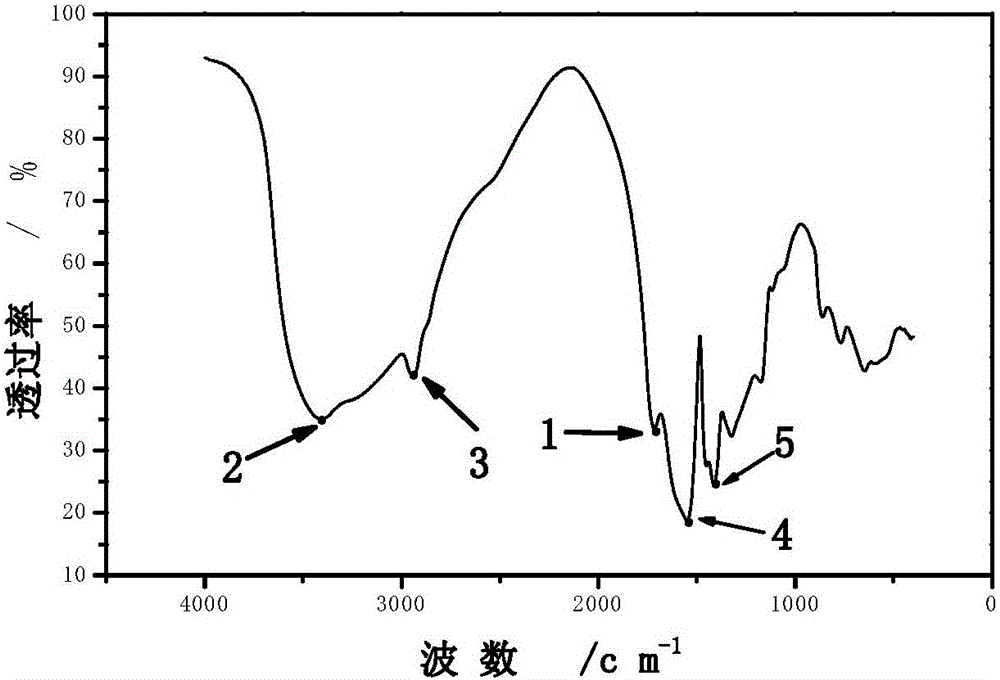
[0141] Ⅲ. For the material characterization results in the implementation of 1 "Step 4" "Relevant characterization of the dry product powder", due to the change of the synthetic formula, the physical and chemical properties of the material change; the characteristics of ...
Embodiment 3
[0146] Step, operation and characterization method are identical with implementation 1; The difference between condition and characterization result and embodiment 1 is:
[0147] Ⅰ. Change the formula in the "third step" and "mixing" of Implementation 1 to: Add 5.909 kg of B component to 1 kg of A component, add 8.927 kg of C component, add 1.364 kg of D component and add 1.364 kg of E component Kilogram.
[0148] Ⅱ. Change the "replacement ratio" in the "replacement" in the "fifth step" of implementation 1 to: the dry product powder of zinc polyacrylic acid reticular cross-linked accounts for 3wt.% of the original zinc oxide feed. The particle size selection of dry powder is changed to 350 mesh ± 50 mesh.
[0149] Ⅲ. For the material characterization results in the implementation of 1 "Step 4" "Relevant characterization of the dry product powder", due to the change of the synthetic formula, the physical and chemical properties of the material change; the characteristics of t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com