A stable long-acting preparation of paliperidone palmitate
A technology of paliperidone and palmitic acid, applied in the field of medicine, can solve the problems of affecting the release and absorption process of the product, slowing the release rate, increasing the particle size, etc., achieving good re-suspension, overcoming the increase in particle size and Effects of reduced release and good dispersibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
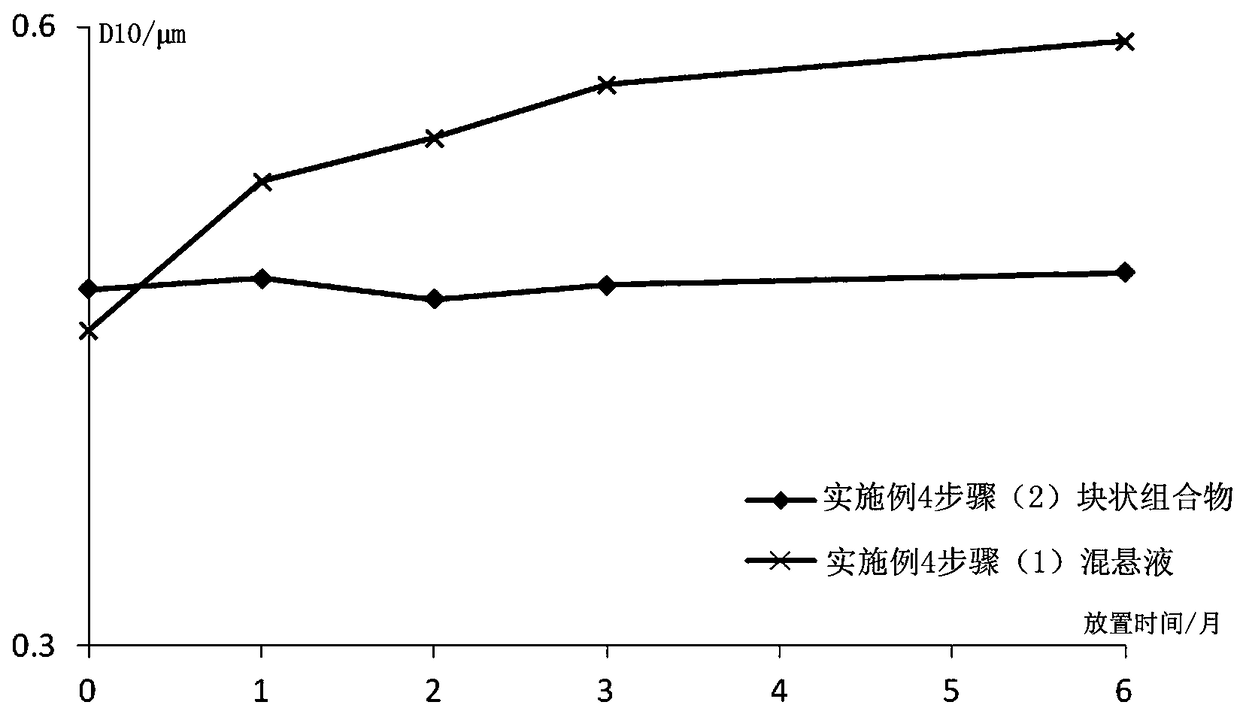
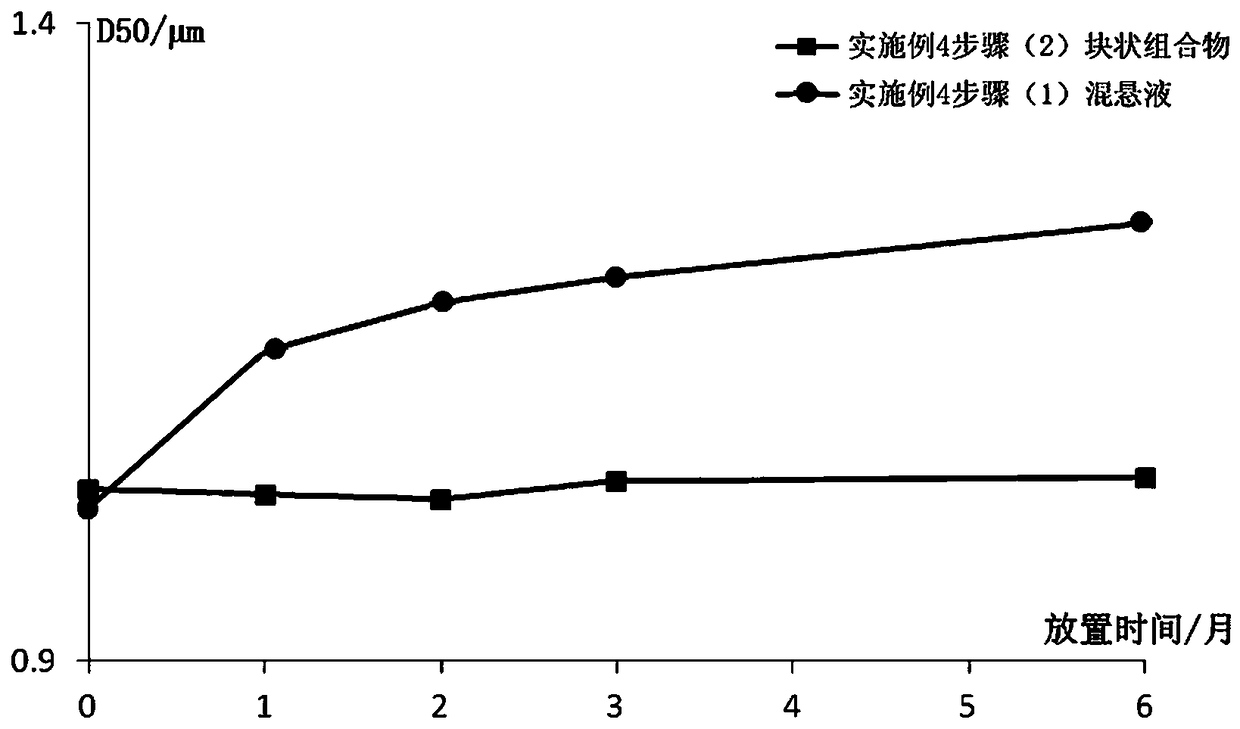
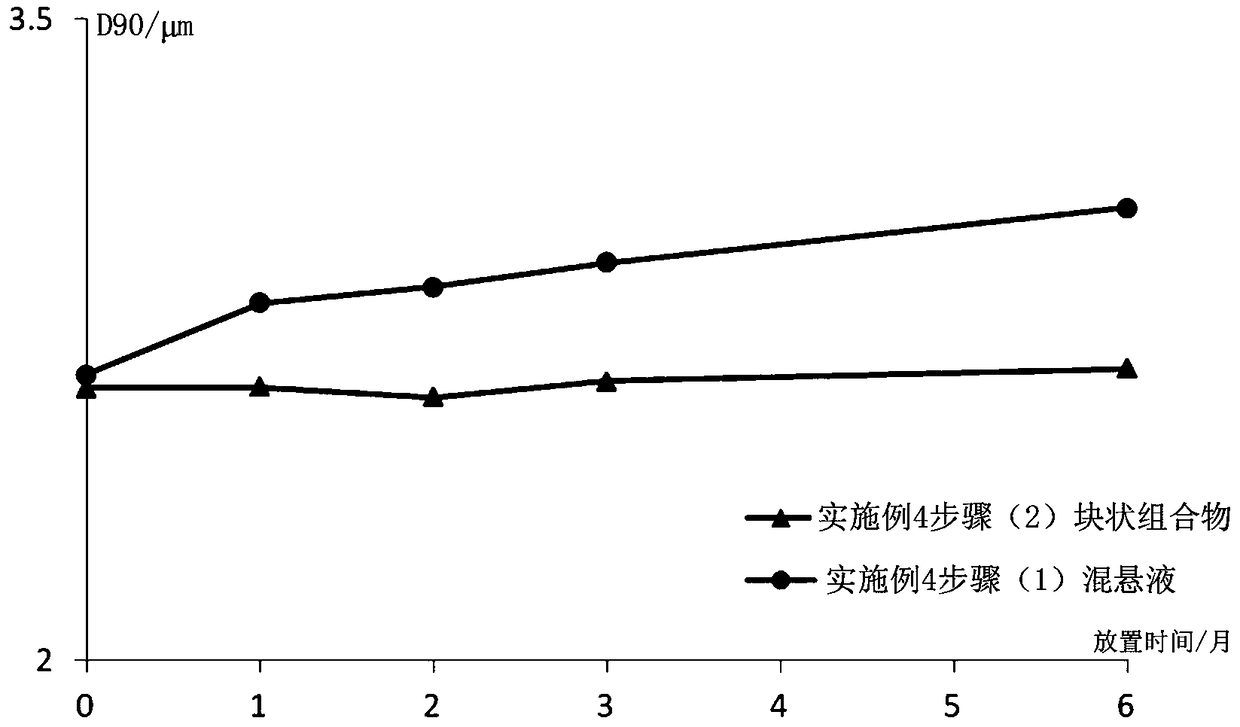
Image
Examples
Embodiment 1
[0081] Example 1 Preparation of Paliperidone Palmitate Block Composition (Specification: 312mg / ml)
[0082] Prescription: Paliperidone Palmitate 312g
[0083] Polysorbate 80 for Injection 15g
[0084] Sodium Carboxymethyl Cellulose 12g
[0085] Mannitol 40g
[0086] Sodium dihydrogen phosphate 0.9
[0087] Sodium hydroxide to adjust the pH to 7
[0088] Add water for injection* to 1000ml;
[0089] (*Used in recipe but removed during processing)
[0090] Preparation Process:
[0091] (1) Preparation of Paliperidone Palmitate Sterile Suspension
[0092] A sterile jet mill was used to control the crushing pressure to 3-5 bar, and the sterile paliperidone palmitate was crushed to an effective particle size of 2-15 μm. Weigh 12g of sodium carboxymethylcellulose, put it in about 500ml of water for injection, swell and dissolve; then take 15g of polysorbate 80 for injection, 40g of mannitol, 0.9g of sodium dihydrogen phosphate, stir and dissolve, and dissolve with 0.5M hydro...
Embodiment 2
[0099] Example 2 Preparation of Paliperidone Palmitate Sterile Block Composition (Specification: 312mg / ml )
[0100] Prescription: Paliperidone Palmitate 312g
[0101] Polysorbate 80 for Injection 15g
[0102] Sodium Carboxymethyl Cellulose 12g
[0103] Mannitol 40g
[0104] Sodium dihydrogen phosphate 0.9g
[0105] NaOH Adjust pH to 7.0
[0106] Add water for injection* to 1000ml
[0107] (*Used in recipe but removed during processing)
[0108] Preparation Process:
[0109] (1) Preparation of Paliperidone Palmitate Sterile Suspension
[0110] Weigh 12g sodium carboxymethylcellulose, put it in about 500ml water for injection, swell and dissolve; then take 15g polysorbate 80 for injection, 40g mannitol, 0.9g sodium dihydrogen phosphate, stir and dissolve, and dissolve with 0.5M hydrogen The sodium oxide solution was used to adjust the pH to 7.0. Weigh 312g of sterile paliperidone palmitate, mix and shear until uniformly dispersed, add water for injection, and adjus...
Embodiment 3
[0117] Example 3 Preparation of Paliperidone Palmitate Sterile Block Composition (Specification: 156mg / ml)
[0118] Prescription: Sterile Paliperidone Palmitate 156g
[0119] Polysorbate 80 for Injection 10g
[0120] Sodium Carboxymethyl Cellulose 8g
[0121] Mannitol 30g
[0122] Sodium dihydrogen phosphate 2.5g
[0123] NaOH Adjust pH to 7.0
[0124] Add water for injection* to 1000ml
[0125] (*Used in recipe but removed during processing)
[0126] Preparation Process:
[0127] (1) Preparation of Paliperidone Palmitate Sterile Suspension
[0128] Weigh 8g of sodium carboxymethylcellulose, put it in about 500ml of water for injection, swell and dissolve; then take 10g of polysorbate 80 for injection, 30g of mannitol, and 2.5g of sodium dihydrogen phosphate, stir and dissolve, and dissolve with 0.5M hydrogen The sodium oxide solution was used to adjust the pH to 7.0. Weigh 156g of sterile paliperidone palmitate and add it to the above solution, stir and shear unti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com